| Mar 02, 2023 |
|
|
|
(Nanowerk News) Molecular clusters are aggregates of molecules that are held together non-covalently, by relatively weak forces. When these clusters are excited, normally one would expect the cluster to simply break apart. However, a fascinating question is whether one can find a way to join the molecules within the cluster together and form chemical bonds between them, before the cluster evaporates.
|
|
This process, called intra-cluster bond formation (ICBF) has been discussed in recent years as a mechanism for the formation of complex molecules in in interstellar medium, as well as a means of forming the complex biomolecules needed for the evolution of life. For example, can one form peptide bonds between amino acids within a cluster? If so, such a mechanism can be instrumental for understanding the abiotic formation of the first peptides and proteins.
|
|
The most widely studied amino acid clusters are those of serine, due to their remarkable properties, such as their propensity to form magic clusters containing exactly eight molecules. These studies have led to much speculation about the role of serine clusters in the origin of life. However, despite decades of research, there have been no observations of bond formation within these clusters.
|
|
One study, led by the Toker group from Bar-Ilan University in Israel, observed peptide bond formation in clusters containing four serine dipeptides that were heated up by collisions. However, they found no evidence for the same process occurring in serine clusters. In that work they concluded that if two serine molecules can bind together to form a dipeptide, then the next stages of polymerization could probably occur readily.
|
|
A new study (Angewandte Chemie, “Peptide Bond Formation in Protonated Serine Dimer Following VUV Photon-Induced Excitation”) led by the Toker group, in collaboration with the group of Patrick Rousseau from Caen University, the group Sergio Diaz Tenderro from Madrid University and that of Laurent Nahon from SOLEIL, provides exactly such a mechanism.
|
 |
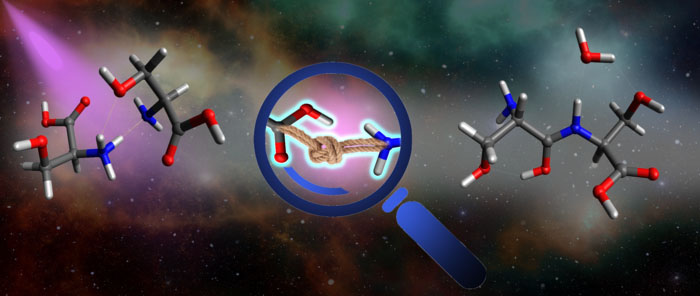
| An illustration of intracluster bond formation: a cluster containing two biomolecules is exposed to UV light (left), which drives a chemical bond to form between the two molecules (center), resulting in a larger and more complex biomolecule (right). (Image: Dario Barreiro Lage, Madrid University)
|
|
“The dream of photo-chemistry is to use light to drive chemical reactions that are not likely to occur otherwise. The present experiments show a beautiful example of such a process,” says Prof. Yoni Toker, of the Department of Physics at Bar-Ilan University.
|
|
The experiment was performed in the French DESIRS beamline in SOLEIL synchrotron, which offers the unique opportunity of combining tunable monochromatic light in the vacuum ultra-violet region with an ion-trap based mass spectrometer. This allows one to produce and select the clusters of interest, irradiate them by synchrotron light, and measure the resulting fragments. Moreover, one can perform even more complex experiments where the fragments are mass-selected and then collisionally excited.
|
|
“We were hoping to see some evidence of bond formation in large serine clusters, such as the magic octamer,” says Ori Licht, from the Toker Lab and the paper’s lead author. “But we were surprised to see bond formation occurring in clusters containing just two serine molecules after the absorption of a UV photon. We were even able to use MS3 measurements to confirm that the fragments we observed are indeed products of intra-cluster bond formation.”
|
|
By comparing measurements of the serine dimer to that of the serine dipeptide, the team found compelling evidence that peptide bond formation had occurred. The measurements were complemented by state-of-the-art quantum chemical calculations, carried out by the group of Sergio Diaz Tendero, of Madrid University. They managed to calculate the excited state dynamics of the cluster, and found that some of the electronic states evolve towards peptide bond formation.
|
|
“In other words, our work provides both the experimental and theoretical framework for how one of the most important biophysical reactions can be triggered abiotically inside molecular clusters by the absorption of ultra-violet light,” says Toker.
|
|
One of the fascinating aspects of biomolecules is their chirality – their handiness. When molecules are synthesized in the lab an equal amount of left-handed or right-handed molecules are produced. This is not the case in living systems, where, for example, all amino acids are left-handed. In follow-up studies the researchers hope to determine whether the intra-cluster bond formation process they observed has a chiral preference: do left-handed molecules bond better with other left-handed molecules, or do they prefer to bond with right-handed molecules?
|