Home > Press > Zinc transporter has built-in self-regulating sensor: New cryo-EM structure of a zinc-transporter protein reveals how this molecular machine functions to regulate cellular levels of zinc, an essential micronutrient
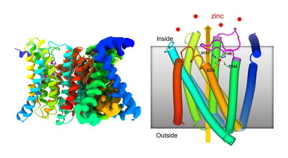 |
| Overall structure of a transmembrane ZIP zinc transporter protein determined by cryo-electron microscopy (cryo-EM) (left) and a schematic showing some of the functional features (right). A flexible loop (magenta) facing into the cell binds to zinc and folds to block the entry of more zinc when levels of this micronutrient get too high.
CREDIT Brookhaven National Laboratory |
Abstract:
Scientists at the U.S. Department of Energys (DOE) Brookhaven National Laboratory have determined the atomic-level structure of a zinc-transporter protein, a molecular machine that regulates levels of this crucial trace metal micronutrient inside cells. As described in a paper just published in Nature Communications, the structure reveals how the cellular membrane protein shifts its shape to move zinc from the environment into a cell, and temporarily blocks this action automatically when zinc levels inside the cell get too high.
Zinc transporter has built-in self-regulating sensor: New cryo-EM structure of a zinc-transporter protein reveals how this molecular machine functions to regulate cellular levels of zinc, an essential micronutrient
Upton, NY | Posted on June 9th, 2023
Zinc is important for many biological activities, but too much can be a problem, said Qun Liu, the Brookhaven Lab biophysicist who led the project. During evolution, different organisms have evolved in many ways to regulate zinc. But no one has shown that a transporter that controls the uptake of zinc from the environment can regulate its own activity. Our study is the first to show a zinc transporter with such a built-in sensor.
The research was conducted as part of Brookhaven Labs Quantitative Plant Sciences Initiative (QPSI). Using a bacterial version of a zinc transporter that shares essential features with zinc transporters in plants, the scientists gained key insights into how these proteins work.
This research is part of our effort to understand how micronutrients like zinc are taken up by plants so we can understand how to design plants that are better able to grow on marginal land for the production of bioenergy, said Brookhaven Lab Biology Department Chair John Shanklin, a co-author on the paper.
The research could also suggest ways to engineer food crops with increased zinc content to improve their nutritional value, the scientists noted.
Cryo-EM plus computation
To solve the protein structure, the Brookhaven team used cryo-electron microscopy (cryo-EM) at the Laboratory for BioMolecular Structure (LBMS). With this technique, scientists can sample many different conformations of a protein instead of a single, crystallized form. Thats important because, in nature, proteins are dynamic, not static; pieces of them move around.
Cryo-EM does not require proteins to form crystals, so we can actually capture dynamic steps that may not feasible using x-ray crystallography, another technique for studying protein structures, Liu said. In essence, with cryo-EM, we can capture more frames of the movie to get a structure that is very helpful in understanding a proteins biological function.
To sort through the many variations in structure, the scientists need powerful computational tools. These include artificial intelligence approaches that use machine learning, some of which Liu has developed. Using these algorithms, the scientists can semi-automatically select and sort through millions of cryo-EM images to find groups of structures with similarities. The method allows them to achieve the highest feasible resolution, and thus to reveal atomic-scale details of the structure.
For this study, this cryo-EM approach revealed key features of one stage of a ZIP (Zrt-/Irt-like protein) zinc transporter that reveals how it regulates its own zinc-uptake activity depending on how much zinc is already in the cell.
Our new data made us revise the previous views as to how this protein works, Liu said.
Tilt to enter, sense to stop
An earlier report based on x-ray crystallography and coevolutional analyses suggested that the transporter may function as a sort of elevator to transport zinc. The new research shows how interactions with zinc on either side of the cellular membrane trigger the movement of parts of the protein to bring zinc into the celland, crucially, block its entry when the levels inside get too high.
Our key structure shows that when the zinc level inside the cell rises to a certain levelbeyond whats required to meet the cells demandsthe excess zinc binds to a loop on the inside of the membrane, Liu said. Then, as this flexible loop reorients, it folds back on itself, and binds in a manner that blocks zinc from entering the cell.
Its almost like the plug going into a bathtub drain and blocking it, Shanklin added.
The scientists also worked out how other parts of the protein move to allow zinc to enter.
When zinc levels inside the cell are low, zinc falls off the loop portion and the plug pops back out of the transporter. Zinc from the environment can move into the transporter. Inside the transporter, the zinc causes part of the protein machine to move up and tilt, closing the exit to the external environment. Once the zinc moves into the cell, the machine will reset itself to work again.
Our cryo-EM structure is the first to show how this loop domain of the protein modulates the transporters activity by feedback depending on the level of zinc, Liu said.
Its also the first structure to show that this zinc transporter is an arrangement of two identical proteinsknown as a dimer. It requires two molecules to do the work, Liu said.
The scientists think that having two molecules acting in the form of a dimer may be related to its function or stabilitywhich theyll explore with future computational simulations of how the molecules work together.
This research could enable new ways of engineering zinc transporters in microbes and plants to optimize their growth in conditions where zinc is too low or too high, potentially on marginal lands for the production of bioenergy and bioproducts, Liu said.
This research was funded by the DOE Office of Science, Office of Biological and Environmental Research (BER) through QPSI, with protein expression, purification, and sample preparation supported by the Office of Basic Energy Sciences (BES). LBMS operations are supported by BER.
####
About DOE/Brookhaven National Laboratory
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
Follow @BrookhavenLab on Twitter or find us on Facebook.
For more information, please click here
Contacts:
Karen McNulty Walsh
DOE/Brookhaven National Laboratory
Office: 631-344-8350
Copyright © DOE/Brookhaven National Laboratory
If you have a comment, please Contact us.
Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
News and information
![]()
Single quantum bit achieves complex systems modeling June 9th, 2023
![]()
Quantum materials: Electron spin measured for the first time June 9th, 2023
![]()
Liquid metal sticks to surfaces without a binding agent June 9th, 2023
![]()
Graphene-based Carbocatalysts: Synthesis, Properties, and ApplicationsBeyond Boundaries June 9th, 2023
Govt.-Legislation/Regulation/Funding/Policy
![]()
Breaking through the limits of stretchable semiconductors with molecular brakes that harness light June 9th, 2023
![]()
Researchers at Purdue discover superconductive images are actually 3D and disorder-driven fractals May 12th, 2023
![]()
Semiconductor lattice marries electrons and magnetic moments March 24th, 2023
Possible Futures
![]()
USTC enhances fluorescence brightness of single silicon carbide spin color centers June 9th, 2023
![]()
Single quantum bit achieves complex systems modeling June 9th, 2023
![]()
Advances in nanotechnology application in biosafety materials A crucial response to COVID-19 pandemic June 9th, 2023
![]()
Researchers discover materials exhibiting huge magnetoresistance June 9th, 2023
Nanomedicine
![]()
Advances in nanotechnology application in biosafety materials A crucial response to COVID-19 pandemic June 9th, 2023
![]()
Nanonitrator: novel enhancer of inorganic nitrate protective effects, predicated on swarm learning approach May 12th, 2023
![]()
Nanobiotechnology: How Nanomaterials Can Solve Biological and Medical Problems April 14th, 2023
![]()
Implantable device shrinks pancreatic tumors: Taming pancreatic cancer with intratumoral immunotherapy April 14th, 2023
Discoveries
![]()
When all details matter — Heat transport in energy materials June 9th, 2023
![]()
Advances in nanotechnology application in biosafety materials A crucial response to COVID-19 pandemic June 9th, 2023
![]()
Researchers discover materials exhibiting huge magnetoresistance June 9th, 2023
![]()
Breaking through the limits of stretchable semiconductors with molecular brakes that harness light June 9th, 2023
Announcements
![]()
Liquid metal sticks to surfaces without a binding agent June 9th, 2023
![]()
Graphene-based Carbocatalysts: Synthesis, Properties, and ApplicationsBeyond Boundaries June 9th, 2023
![]()
When all details matter — Heat transport in energy materials June 9th, 2023
![]()
Advances in nanotechnology application in biosafety materials A crucial response to COVID-19 pandemic June 9th, 2023
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]()
USTC enhances fluorescence brightness of single silicon carbide spin color centers June 9th, 2023
![]()
Single quantum bit achieves complex systems modeling June 9th, 2023
![]()
Advances in nanotechnology application in biosafety materials A crucial response to COVID-19 pandemic June 9th, 2023
![]()
Researchers discover materials exhibiting huge magnetoresistance June 9th, 2023










