(Nanowerk News) Researchers have invented a nano-thin superbug-slaying material that could one day be integrated into wound dressings and implants to prevent or heal bacterial infections.
The innovation – which has undergone advanced pre-clinical trials – is effective against a broad range of drug-resistant bacterial cells, including ‘golden staph’, which are commonly referred to as superbugs.
Antibiotic resistance is a major global health threat, causing about 700,000 deaths annually, a figure which could rise to 10 million deaths a year by 2050 without the development of new antibacterial therapies.
The new study led by RMIT University and the University of South Australia (UniSA) tested black phosphorus-based nanotechnology as an advanced infection treatment and wound healing therapeutic.
Results published in Advanced Therapeutics (“Layered Black Phosphorus Nanoflakes Reduce Bacterial Burden and Enhance Healing of Murine Infected Wounds”) show it effectively treated infections, killing over 99% of bacteria, without damaging other cells in biological models.
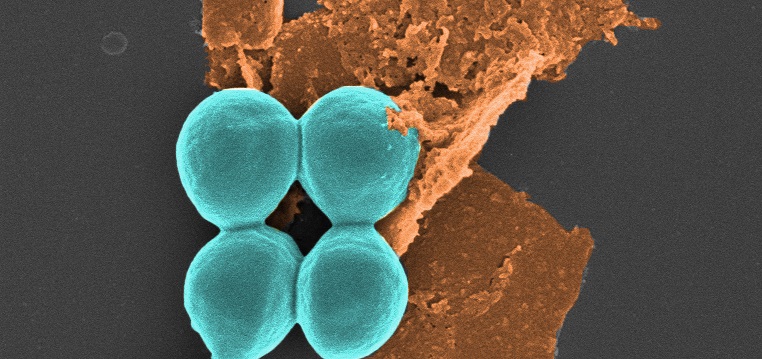
 The ball shapes are bacteria and the “sheet” is black phosphorus, under the microscope at RMIT University. (Note these images have been coloured in post-production.) (Image: Aaron Elbourne and colleagues, RMIT University
The treatment achieved comparable results to an antibiotic in eliminating infection and accelerated healing, with wounds closing by 80% over seven days.
The superbug-killing nanotechnology developed internationally by RMIT was rigorously tested in pre-clinical trials by wound-healing experts at UniSA. RMIT has sought patent protection for the black phosphorus flakes including its use in wound healing formulations, including gels.
RMIT co-lead researcher, Professor Sumeet Walia, said the study showed how their innovation provided rapid antimicrobial action, then self-decomposed after the threat of infection had been eliminated.
“The beauty of our innovation is that it is not simply a coating – it can actually be integrated into common materials that devices are made of, as well as plastic and gels, to make them antimicrobial,” said Walia from RMIT’s School of Engineering.
A previous study led by RMIT revealed that black phosphorus was effective at killing microbes when spread in nano-thin layers on surfaces used to make wound dressings and implants such as cotton and titanium, or integrated into plastics used in medical instruments.
The ball shapes are bacteria and the “sheet” is black phosphorus, under the microscope at RMIT University. (Note these images have been coloured in post-production.) (Image: Aaron Elbourne and colleagues, RMIT University
The treatment achieved comparable results to an antibiotic in eliminating infection and accelerated healing, with wounds closing by 80% over seven days.
The superbug-killing nanotechnology developed internationally by RMIT was rigorously tested in pre-clinical trials by wound-healing experts at UniSA. RMIT has sought patent protection for the black phosphorus flakes including its use in wound healing formulations, including gels.
RMIT co-lead researcher, Professor Sumeet Walia, said the study showed how their innovation provided rapid antimicrobial action, then self-decomposed after the threat of infection had been eliminated.
“The beauty of our innovation is that it is not simply a coating – it can actually be integrated into common materials that devices are made of, as well as plastic and gels, to make them antimicrobial,” said Walia from RMIT’s School of Engineering.
A previous study led by RMIT revealed that black phosphorus was effective at killing microbes when spread in nano-thin layers on surfaces used to make wound dressings and implants such as cotton and titanium, or integrated into plastics used in medical instruments.