Home > Press > New designs for solid-state electrolytes may soon revolutionize the battery industry: Scientists achieve monumental improvements in lithium-metal-chloride solid-state electrolytes
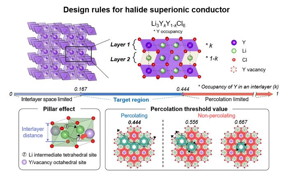 |
| The arrangement of metal ions (yttrium in this case) within each layer affects the ionic conductivity. To ensure the unobstructed movement of lithium ions, the number of metal ions occupying available sites within each layer should be less than 0.444. Furthermore, to create a sufficiently wide pathway for lithium ions within each layer, the occupancy of metal ions should be more than 0.167. Therefore, achieving an occupancy of metal ions between 0.167 and 0.444 within each layer results in a conductive layer with high ionic conductivity.
CREDIT Institute for Basic Science |
Abstract:
Researchers led by Professor KANG Kisuk of the Center for Nanoparticle Research within the Institute for Basic Science (IBS), have announced a major breakthrough in the field of next-generation solid-state batteries. It is believed that their new findings will enable the creation of batteries based on a novel chloride-based solid electrolyte that exhibits exceptional ionic conductivity.
New designs for solid-state electrolytes may soon revolutionize the battery industry: Scientists achieve monumental improvements in lithium-metal-chloride solid-state electrolytes
Daejeon, Republic of Korea | Posted on November 3rd, 2023
A pressing concern with current commercial batteries their reliance on liquid electrolytes, which leads to flammability and explosion risks. Therefore, the development of non-combustible solid electrolytes is of paramount importance for advancing solid-state battery technology. As the world gears up to regulate internal combustion engine vehicles and expand the use of electric vehicles in the ongoing global shift toward sustainable transportation, research into the core components of secondary batteries, particularly solid-state batteries, has gained significant momentum.
To make solid-state batteries practical for everyday use, it is crucial to develop materials with high ionic conductivity, robust chemical and electrochemical stability, and mechanical flexibility. While previous research successfully led to sulfide and oxide-based solid electrolytes with high ionic conductivity, none of these materials fully met all these essential requirements.
In the past, scientists have also explored chloride-based solid electrolytes, known for their superior ionic conductivity, mechanical flexibility, and stability at high voltages. These properties led some to speculate that chloride-based batteries are the most likely candidates for solid-state batteries. However, these hopes quickly died out, as the chloride batteries were considered impractical due to their heavy reliance on expensive rare earth metals, including yttrium, scandium, and lanthanide elements, as secondary components.
To address these concerns, the IBS research team looked at the distribution of metal ions in chloride electrolytes. They believed the reason trigonal chloride electrolytes can achieve low ionic conductivity is based on the variation of metal ion arrangements within the structure.
They first tested this theory on lithium yttrium chloride, a common lithium metal chloride compound. When the metal ions were positioned near the pathway of lithium ions, electrostatic forces caused obstruction in their movement. Conversely, if the metal ion occupancy was too low, the path for lithium ions became too narrow, impeding their mobility.
Building on these insights, the research team introduced strategies to design electrolytes in a way that mitigates these conflicting factors, ultimately leading to the successful development of a solid electrolyte with high ionic conductivity. The group went further to successfully demonstrate this strategy by creating a lithium-metal-chloride solid-state battery based on zirconium, which is far cheaper than the variants that employ rare earth metals. This was the first instance where the significance of the metal ions arrangement on a materials ionic conductivity was demonstrated.
This research brings to light the often-overlooked role of metal ion distribution in the ionic conductivity of chloride-based solid electrolytes. It is expected that the IBS Centers research will pave the way for the development of various chloride-based solid electrolytes and further drive the commercialization of solid-state batteries, promising improved affordability and safety in energy storage.
Corresponding author KANG Kisuk states, This newly discovered chloride-based solid electrolyte is poised to transcend the limitations of conventional sulfide and oxide-based solid electrolytes, bringing us one step closer to the widespread adoption of solid-state batteries.
This research was published on November 3, 2023, in Science, which is one of the world’s most prestigious scientific journals.
####
For more information, please click here
Contacts:
William Suh
Institute for Basic Science
Office: 82-010-379-37830
Copyright © Institute for Basic Science
If you have a comment, please Contact us.
Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
News and information
![]()
Nanoparticle quasicrystal constructed with DNA: The breakthrough opens the way for designing and building more complex structures November 3rd, 2023
![]()
What a 2D quantum superfluid feels like to the touch November 3rd, 2023
Possible Futures
![]()
Light guide plate based on perovskite nanocomposites November 3rd, 2023
![]()
Charged molecular beasts the basis for new compounds: Researchers at Leipzig University use aggressive fragments of molecular ions for chemical synthesis November 3rd, 2023
![]()
Study on Magnetic Force Microscopy wins 2023 Advances in Magnetism Award: Analysis of finite size effects reveals significant consequences for density measurements November 3rd, 2023
![]()
Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
Discoveries
![]()
Nanoparticle quasicrystal constructed with DNA: The breakthrough opens the way for designing and building more complex structures November 3rd, 2023
![]()
What a 2D quantum superfluid feels like to the touch November 3rd, 2023
Announcements
![]()
Light guide plate based on perovskite nanocomposites November 3rd, 2023
![]()
Charged molecular beasts the basis for new compounds: Researchers at Leipzig University use aggressive fragments of molecular ions for chemical synthesis November 3rd, 2023
![]()
Study on Magnetic Force Microscopy wins 2023 Advances in Magnetism Award: Analysis of finite size effects reveals significant consequences for density measurements November 3rd, 2023
![]()
Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]()
Nanoparticle quasicrystal constructed with DNA: The breakthrough opens the way for designing and building more complex structures November 3rd, 2023
![]()
What a 2D quantum superfluid feels like to the touch November 3rd, 2023
Automotive/Transportation
![]()
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]()
Chloride ions from seawater eyed as possible lithium replacement in batteries of the future August 11th, 2023
![]()
Graphene-based Carbocatalysts: Synthesis, Properties, and ApplicationsBeyond Boundaries June 9th, 2023










