Definition:
‘the branch of technology that deals with dimensions and tolerances of less than 100 nanometers, especially the manipulation of individual atoms and molecules.’
Nanotechnology is science, engineering, and technology conducted at the nanoscale, which is about 1 to 100 nanometers.
Physicist Richard Feynman, the father of nanotechnology.
Nanoscience and nanotechnology are the study and application of extremely small things and can be used across all the other science fields, such as chemistry, biology, physics, materials science, and engineering.
The ideas and concepts behind nanoscience and nanotechnology started with a talk entitled “There’s Plenty of Room at the Bottom” by physicist Richard Feynman at an American Physical Society meeting at the California Institute of Technology (CalTech) on December 29, 1959, long before the term nanotechnology was used. In his talk, Feynman described a process in which scientists would be able to manipulate and control individual atoms and molecules. Over a decade later, in his explorations of ultraprecision machining, Professor Norio Taniguchi coined the term nanotechnology. It wasn’t until 1981, with the development of the scanning tunnelling microscope that could “see” individual atoms, that modern nanotechnology began.

Medieval stained glass windows are an example of how nanotechnology was used in the pre-modern era.
It’s hard to imagine just how small nanotechnology is. One nanometer is a billionth of a meter or 10-9 of a meter. Here are a few illustrative examples:
There are 25,400,000 nanometers in an inch
A sheet of newspaper is about 100,000 nanometers thick
On a comparative scale, if a marble were a nanometer, then one meter would be the size of the Earth
Nanoscience and nanotechnology involve the ability to see and to control individual atoms and molecules. Everything on Earth is made up of atoms—the food we eat, the clothes we wear, the buildings and houses we live in, and our own bodies.
But something as small as an atom is impossible to see with the naked eye. In fact, it’s impossible to see with the microscopes typically used in high school science classes. The microscopes needed to see things at the nanoscale were invented relatively recently—about 30 years ago.
Once scientists had the right tools, such as the scanning tunnelling microscope (STM) and the atomic force microscope (AFM), the age of nanotechnology was born.
Although modern nanoscience and nanotechnology are quite new, nanoscale materials were used for centuries. Alternate-sized gold and silver particles created colours in the stained glass windows of medieval churches hundreds of years ago. The artists back then just didn’t know that the process they used to create these beautiful works of art actually led to changes in the composition of the materials they were working with.
Today’s scientists and engineers are finding a wide variety of ways to deliberately make materials at the nanoscale to take advantage of their enhanced properties such as higher strength, lighter weight, increased control of light spectrum, and greater chemical reactivity than their larger-scale counterparts.
Self-cleaning I – the “Lotus-Effect”
 Figure 1: The lotus plant (Nelumbo nucifera).
Figure 1: The lotus plant (Nelumbo nucifera).
The lotus plant above (Nelumbo nucifera) is revered in Asia for its exceptional cleanness. Although it grows in muddy waters, its leaves always appear immaculately clean. The plants’ leaves are superhydrophobic, i.e. drops of water roll off free of residue, taking any impurities with them.
The lotus plant (Nelumbo nucifera)
Figure 1: The lotus plant (Nelumbo nucifera).Investigations into the surface using reflection electron microscopy (REM) have shown that the surface of the leaf is not especially even, which you might intuitively assume, but has instead a special, characteristic roughness
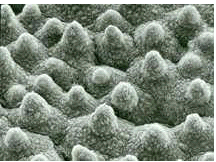
Figure 2: Electron microscope photograph of the surface of a lotus flower leaf. The combination of surface roughness and water-repellent wax cristals gives it superhydrophobic properties.
(Figure 2): Systematically arranged, water-repellent, nano-size wax crystals form three-dimensional structures, similar to small nipples, which are no greater than a few nanometers or micrometres in size.
When combined with the waxes’ water-repellent chemical properties, these structures make the lotus leaf extremely non-wettable, a state called ultra hydrophobic or super-hydrophobic, and they give it its self-cleaning properties. Dirt particles only sit on the tip of the wax crystals and as a result, only a very small surface area comes into contact with the planet’s surface. If water falls onto a leaf surface like this, the interplay of the surface tension and the low attraction force between the surfaces and the water produce a spherical water drop which only sits on the tips of the wax structures. If the leaf tips in the slightest, the water drop immediately rolls off, taking the dirt particles with it (Figure 3). As the gravitational pull between the dirt and the leaf’s surface is very slight, i.e. it is smaller than that between the water and the dirt, even lipophilic impurities, such as soot, for example, can be washed away.
In most cases, this form of self-cleaning serves not so much to protect the plant from dirt as from pathogens (e.g. fungal spores, bacteria). Super-hydrophobia is a property which is not only found in the lotus plant however, it is present in around 300 other plant species as well. Insects too, such as dragonflies and butterflies have this property on the surface of their wings.
The combination of surface roughness and water-repellent wax cristals gives it superhydrophobic properties. The principle of self-cleaning was discovered in 1973 by the botanist Wilhelm Barthlott and his team at the University of Bonn. As it is a purely physio-chemical effect that is not bound to a living system, Barthlott believed that its technical implementation should be possible. The industry, however, showed little interest initially, and so he produced his own technical surfaces with these properties, applied for a patent for his invention2 and registered the brand name “Lotus-Effect®”.
The first commercial product made in collaboration with industry was a silicone resin house paint which has since become widely used and in which silicon nanoparticles form micro-structured surface3.
Other “Lotus-Effect?” products include ceramic roof tiles, architectural glass and a spray for industrial use which makes surfaces temporarily non-wettable and self-cleaning4.
Advances are also being made in the development of engineered fabrics that are self-cleaning.
Composite materials made up of nanoparticles in a coating matrix make it possible to manufacture the surface structure required for the “Lotus-Effect”.
Environment and health
Self-cleaning or easy to clean surfaces can reduce the amount of cleaning required. In the case of industrial cleaning, in particular, it can reduce labour costs and extend a material’s durability. Lower energy costs and less use of cleaning detergents are expected to be the primary environmental benefits.
