| Nov 15, 2022 |
|
(Nanowerk News) A new Australian-led stud finds gold atoms could be key to unlocking organic reactions.
|
|
Organic molecules are the building blocks for materials we use every day – from our clothes and coffee cups to the screen displays of our phones. Controlling reactions of these organic molecules is the key to designing materials with functional properties.
|
|
Reactions targeting carbon-hydrogen (C-H) bonds have long been of scientific interest given that almost all organic molecules contain these bonds. Led by FLEET at Monash University, a new study, published in the Journal of the American Chemical Society (“Selective Activation of Aromatic C-H Bonds Catalysed by Single Gold Atoms at Room Temperature”), finds that individual gold atoms may provide a low energy route for reactions which can target specific C-H bonds.
|
 |
| Organic (DCA) molecules combine with gold atoms (Au) on a silver surface, (Ag). (Image: FLEET)
|
The “holy grail” of chemical reactions
|
|
“One of FLEET’s goals is the development of materials whose electronic properties may be exploited in low-energy technologies,” says corresponding author A/Prof Agustin Schiffrin.
|
|
Organic molecules may serve as useful building blocks for tuneable construction of these materials, provided reactions between molecules can be controlled at the atomic scale.
|
|
Carbon-hydrogen bonds are among the most common bonds in organic molecules. Because of this, the ability to target specific C-H bonds in chemical reactions has been described by some researchers as the “holy grail”. Unfortunately, two big challenges stand in the way of C-H activation reactions:
|
|
2. Difficulty in targeting only one specific bond for reaction (poor selectivity).
1. A lot of energy is required to break these bonds (high activation energy).
|
 |
| Scanning tunnelling microscopy (a) and non-contact atomic force microscopy (b) at Monash University of bonded DCA molecules and Au atoms allow direct observation of the chemical structure with covalent C-Au bonds. Scale bars: 0.5 nm. (Image: FLEET)
|
All that glitters…?
|
|
The Monash researchers have found that single gold atoms may provide a route to C-H activation.
|
|
The researchers combined small numbers of individual gold atoms with organic 9,10-dicyanoanthracene (DCA) molecules on an atomically flat surface of silver, Ag(111).
|
|
“We used atomic-scale experimental techniques – scanning tunnelling microscopy and atomic force microscopy – to image and characterise the samples,” explains lead author Benjamin Lowe, who is a FLEET PhD student at Monash. “These techniques revealed unusual covalent bonds between the carbon atoms of the DCA molecules and the gold atoms.”
|
|
The formation of such covalent bonds suggested that specific C-H bonds had to have first been broken. Working with theoretical collaborators at the Czech Academy of Sciences, the researchers uncovered a reaction pathway which suggested that a metal-organic intermediate state formed by single gold atoms with pairs of DCA molecules could help such a reaction proceed.
|
|
Significantly, the reaction pathway uncovered can only explain C-H breaking of one specific C-H bond. The researchers found a dramatic decrease of the energy required to break this specific C-H bond (activation barrier), enabling the reaction at room temperature.
|
 |
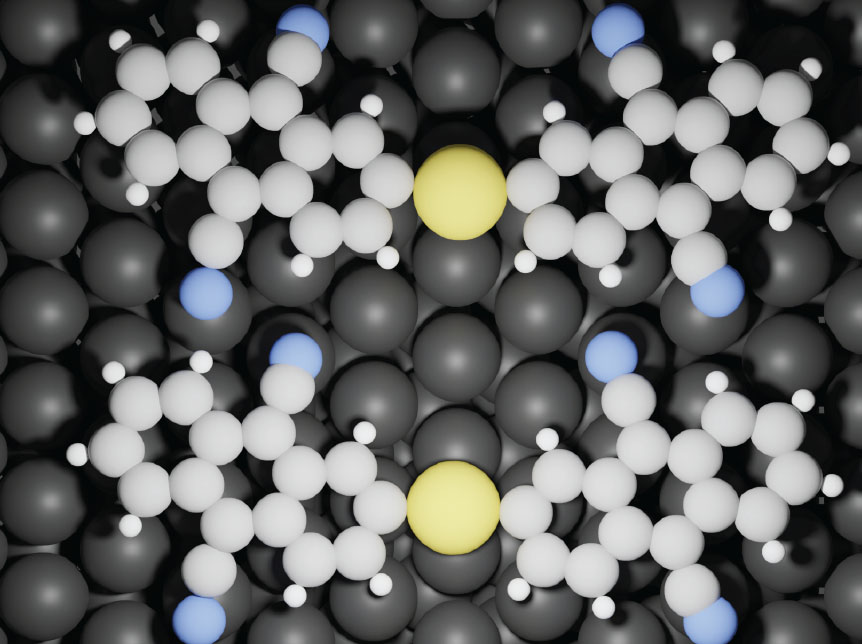
| Gold atoms provide a low-energy reaction route, allowing breaking of C-H bonds at room temperature, and formation of covalently bonded DCA-Au-DCA structures. Grey: C, white: H, blue: N, black: Ag (surface), yellow: Au. (Image: FLEET)
|
|
“This study directly addresses the two biggest challenges – namely poor selectivity and high activation barrier – that limit specific dissociation of C-H bonds in organic molecules,” explains FLEET Chief Investigator Agustin Schiffrin. “Our approach can potentially open the door to the synthesis of novel organic and metal-organic nanomaterials, with properties useful for electronics, optoelectronics, sensing, catalysis, etc.”
|
What’s next?
|
|
Given the broad interest in reactions of organic molecules across a range of fields, this promising reaction has many potential applications such as polymer fabrication and modification of pharmaceutical products.
|
|
At FLEET, the researchers hope to exploit this selective and efficient reaction to produce atomically thin materials with desirable electronic properties.
|