| Mar 05, 2022 |
|
(Nanowerk News) Researchers from Tokyo Metropolitan University have discovered key clues behind the workings of “single-atom” catalysts based on iron-pyridine sites in a carbon matrix (Environmental Science & Technology, “Elucidating the Mechanistic Origin of a Spin State-Dependent FeNx–C Catalyst toward Organic Contaminant Oxidation via Peroxymonosulfate Activation”).
|
 |
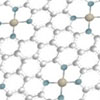
| High-spin iron pyridine states are activated via two different mechanisms, either through the production of hydroxy radicals from peroxymonosulfate, or the formation of a Fe(V)-O complex. (Image: Tokyo Metropolitan University)
|
|
They firstly developed a new, simple synthesis method for a catalyst that activate peroxymonosulfate, highly effective in breaking down pollutants that do not easily biodegrade. They discovered that “high-spin” state iron sites strongly correlated with catalyst performance thanks to two distinct chemical pathways.
|
|
The world is full of useful synthetic chemicals, whether it be household solvents, pharmaceuticals, or fertilizers. But with it has come a similar range of pollutants in our environment, much to the detriment of both ecosystems and our own wellbeing. In particular, a class of pollutants known as “refractory” organics are particularly worrying. They are not easily biodegraded, and stubbornly stick around in the environment for very long periods of time. That’s why effective removal or degradation strategies in wastewater streams are very important.
|
|
Scientists have been trying to develop effective catalysts which help break down harmful refractory pollutants. A promising type of catalyst is the “single-atom” catalyst (SAC), where metal atoms are uniformly dispersed within a matrix of carbon atoms. The incorporation of iron is particularly promising, since the results are cheap, non-toxic, and very effective. Yet despite demonstrations in the lab, SACs remain difficult to produce, and the mechanism by which they work remains unclear.
|
|
Now, a team led by Associate Professor Shiro Kubuki of Tokyo Metropolitan University has successfully developed a simple method to produce SACs with iron-pyridine sites (iron surrounded by four nitrogen atoms) incorporated into carbon sheets. Based on pyrolysis, the decomposition and recombination of chemicals using heat, the team took precursors of iron in complex three-dimensional structures known as metal oxide frameworks (MOFs), ground them together with melamine, and heated them in an inert atmosphere at temperatures exceeding 500 degrees Celsius. When activating peroxymonosulfate, a common oxidizing agent, they were shown to be very effective at removing pollutants such as bisphenol A (BPA), a common chemical in resins and plastics.
|
|
In their work, the team found that catalysts made in different ways had different efficacy. By using an experimental technique called Mossbauer spectroscopy and Density Functional Theory calculation, they studied the state of iron pyridine sites in different batches.
|
|
Interestingly, they found a strong correlation between the presence of “high-spin” Fe(ii) and Fe(iii) states and the effectiveness of the catalyst. “High-spin” refers to the specific way in electrons fill high energy orbitals around the iron; the interaction between the iron and surrounding items helps create “high-spin” and “low-spin” states which behave differently.
|
|
Using computational techniques, they found for the first time that there were in fact two different pathways by which high-spin states could help reactions occur. Fe(ii) sites interacted strongly with peroxymonosulfate to create highly reactive hydroxyl radicals; Fe(iii) sites, on the other hand, formed groups known as Fe(V)-O complexes, which in turn helped break down organic pollutants.
|
|
Combining both mechanistic insights and an easy method of making SACs, the team hopes this technology will see more deployment in real wastewater treatment systems and in efforts to clean up the environment.
|