| Jul 16, 2022 |
|
|
|
(Nanowerk News) For all the excitement about the healing potential of embryonic stem cells, with their ability to mature into any kind of body tissue, these cells are, surprisingly, not the winners of the “endless possibilities” award. This prize goes to even earlier cells that make up the embryo in the first day or two of its existence.
|
|
Researchers at the Weizmann Institute of Science have now revealed how these early cells start giving up their limitless abilities and, no less important, how such abilities can be restored (Molecular Cell, “SUMOylation of linker histone H1 drives chromatin condensation and restriction of embryonic cell fate identity”).
|
|
“We’ve found a new mechanism that can take cells back in time to the very first step in the life of an embryo,” says Dr. Yifat Merbl of the Systems Immunology Department, who coheaded the research team with Prof. Jacob Hanna of the Molecular Genetics Department.
|
|
When an embryo consists of two cells, these cells possess a property called totipotency, or the ability for all things. These cells are capable of transforming into not only all body tissue types but also placenta. Once the embryo reaches the four-cell stage, some of its cells start losing their totipotency and subsequently became pluripotent, or possessing the ability for many things – that is, they can give rise to any type of body tissue, but not to that of the placenta.
|
|
As the process continues, the early embryo develops into a mulberry-like clump of several dozen pluripotent stem cells, which keep dividing and ultimately maturing into nerve, muscle and all other cell types. In nature, of course, this process moves in one direction only, from the fertilized egg to numerous cells that assume more and more specialized tasks. But in research that earned them the Nobel Prize in 2012, scientists showed that in the lab this cellular specialization is reversible: By introducing a few genes into mature cells, they reprogrammed them to revert to an unspecialized, pluripotent state.
|
|
Now, Weizmann researchers have found a novel way to take this reprogramming even further – namely, returning pluripotent embryonic stem cells to a totipotent, two-cell state. The scientists accomplished this feat by manipulating a new kind of mechanism they discovered: a change in the shape of DNA’s chromatin packaging that causes cells to lose their capacity for totipotency, thus beginning to mold their fates and future identities.
|
|
“This mechanism is sort of a lock that helps to keep the development going in one direction,” says Dr. Daoud Sheban, who led the study as part of his PhD studies in Merbl’s and Hanna’s labs. “It physically condenses the chromatin, preventing the cell’s DNA from expressing genes that have been identified with totipotency. Once we reverse the condensation, the chromatin opens up, and this causes the unlocking.”
|
 |
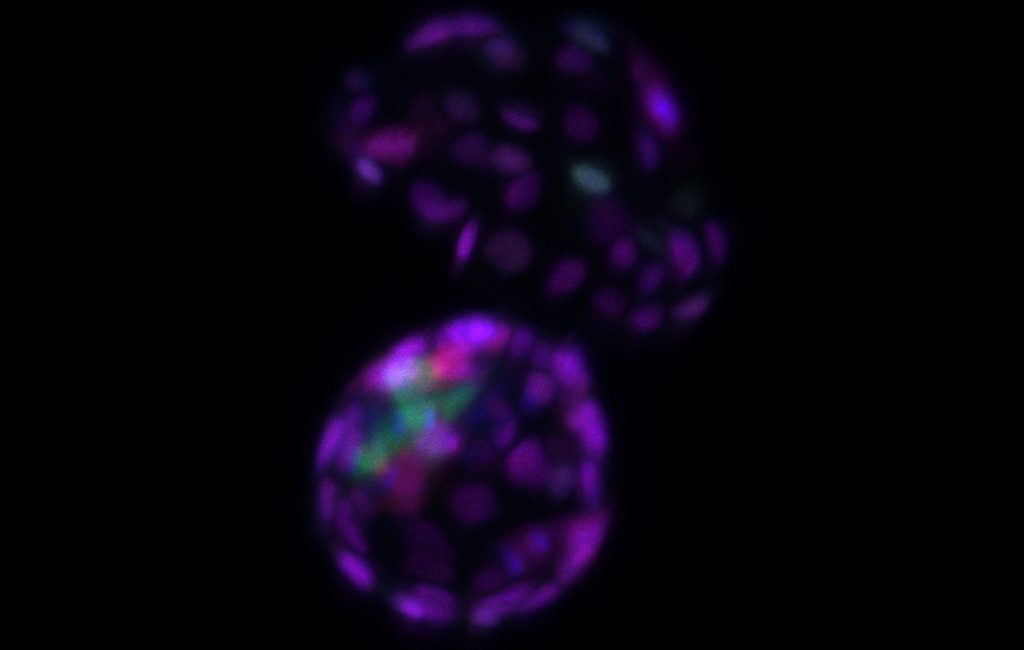
| Following removal of the SUMO tag from proteins, embryonic stem cells reacquire totipotency hallmarks, regaining their ability to form both the embryonic cell lineage (bluish-green) that will give rise to all tissue types in the body and the extraembryonic cell lineage (purple) that will give rise to the future placenta. (Image courtesy of the researchers)
|
|
By experimenting with the embryos and stem cells of mice, Sheban and colleagues revealed how chromatin’s spatial arrangement changes to make it condensed: One of its protein components, called histone H1, is tagged by a protein belonging to a family of small ubiquitin-like modifiers, or SUMO. Instead of marking proteins for degradation, like ubiquitin, its famous cousin, SUMO modifies their function.
|
|
Tagging by SUMO is one of numerous modifications that proteins can undergo at a relatively late stage, namely, once they are fully synthesized. As the case of histone H1 shows, such tagging can have a decisive effect on a protein’s activity. “Regardless of the protein’s levels in the cell, its function can be regulated by late-stage modifications,” Merbl says.
|
|
When the scientists genetically manipulated embryonic stem cells to remove histone H1 proteins or the enzyme responsible for SUMO tagging, these cells reverted to totipotency, regaining their ability to form placental tissue. Not only did their chromatin open up, but more than a hundred genes involved in totipotency were once again expressed.
|
|
The study’s findings open new avenues of research into embryonic development and disease processes, for example, the underlying mechanisms of cancer. Defects in SUMO tagging may be involved in the loss of identity by cancer cells, causing these cells to revert to an immature state characterized by aggressive growth. The study could also have implications for regenerative medicine by shedding new light on ways in which cells mature and specialize. Such an in-depth understanding might, in turn, help bring added precision to the field of growing tissues and organs for therapeutic purposes.
|
|
“Our findings may add a new tool to be used in tissue regeneration – unlocking a cell’s chromatin to create a desired spatial arrangement,” Sheban says.
|