| Apr 07, 2024 |
|
|
|
(Nanowerk News) In an era where the quest for sustainable energy sources has become paramount, researchers are tirelessly exploring innovative avenues to enhance fuel production processes. One of the most important tools in converting chemical energy into electrical energy and vice versa is electrocatalysis, which is already used in various green-energy technologies.
|
|
Electrocatalysis speeds up electrochemical reactions through the use of catalysts – substances that increase reaction rates without being consumed themselves. Electrocatalysis is fundamental in devices like fuel cells and electrolyzers, where it enables the efficient transformation of fuels such as hydrogen and oxygen into electricity, or water into hydrogen and oxygen, respectively, facilitating a cycle of clean energy.
|
|
But the problem is efficiency. Traditional electrocatalysis methods often fall short of maximizing the transport of reactants to the catalyst’s surface, which is a key step in energy conversion. This lowers the reaction’s overall efficiency, and slows down our progress towards clean energy solutions.
|
|
Now, scientists led by Magalí Lingenfelder at EPFL have developed a novel approach to track the fundamental processes that enhance the efficiency of clean fuel production. Published in Nature Communications (“Enhancement of electrocatalysis through magnetic field effects on mass transport”), the work focuses on the promising intersection of magnetic fields and electrocatalysis, offering a pathway to more efficient and environmentally friendly fuel production technologies.
|
 |
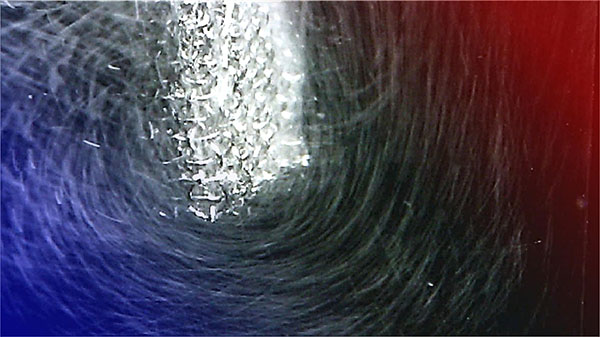
| Visualization of the whirling motion of O2 bubbles around a metal catalyst under a 0.43 Tesla magnetic field, in reaction conditions and at room temperature. (Image: Priscila Vensaus/EPFL)
|
|
The study showed that surrounding the catalysts with magnetic fields create Lorentz forces – the forces that magnetic fields exert on moving electric charges. These in turn induce whirling motions that enhance the movement of reactants and products at the catalyst surface, ensuring a more consistent and rapid reaction but also overcoming the limitations posed by reactant scarcity, a common hurdle in reactions like the oxygen reduction reaction (ORR), critical for fuel cells.
|
|
To do all this, the researchers had to build a tool for observing the movement of ions in real time under a magnetic field, using an advanced magneto-electrochemical setup. For the actual sophisticated setup, Lingenfelder turned to her office neighbor and spintronics expert, Professor Jean-Philippe Ansermet, who had also studied spin effects in electrochemistry.
|
|
“We adapted Jean-Philippe’s electromagnet to measure magnetic field effects on key electrocatalytic reactions for green energy,” she says. “Using a creative trick developed by Priscila and Yunchang [the first authors of the study], we were able to track in situ how ions move in the electrolyte under a magnetic field and to provide a solid ground on how to apply magnetic fields to boost electrocatalysis in a reproducible way”.
|
|
By applying magnetic fields to non-magnetic electrodes and monitoring reactions, the scientists were able to decouple the different effects and observe how magnetic forces can stir and enhance the movement of reactants around the catalyst. This process, akin to creating miniature whirlpools, significantly improves the efficiency of reactions crucial for green hydrogen production, offering a promising avenue for advancing sustainable energy technologies.
|
|
Is the new method practical? In the study, the scientists show more than a 50% boost in activity for the oxygen reduction reaction induced by magnetic fields on non-magnetic interfaces. This represents a substantial jump in efficiency, but, most importantly, allowed the team to resolve many fundamental controversies in the field by demonstrating the mechanisms and conditions needed for magnetic fields to enhance different electrocatalytic reactions involving gas products or reactants like hydrogen and oxygen.
|
|
The study charts the way towards using magnetic fields to improve the efficiency of electrocatalysis that can propel us towards more effective sustainable fuel production. It can revolutionize energy conversion technologies, make fuel cells more widely adopted e.g., in hydrogen vehicles, and increase the production of hydrogen as a clean energy source, also mitigating the impact of our energy consumption on the planet’s climate change.
|