Home > Press > A molecule like a nanobattery: Chemical scientists decipher complex electronic structure of a three-nuclear metallorganic compound with the capacity of donating and receiving multiple electrons
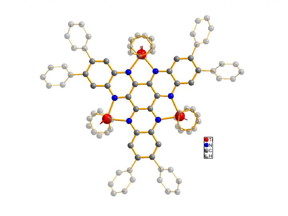 |
| The structure of the molecule under study (titanium shown in red, nitrogen in blue, carbon in grey). The basic body of the molecule is highlighted, whereas hydrogen atoms are hidden for simplification.
CREDIT Graphics: Ruediger Beckhaus / University of Oldenburg |
Abstract:
How do molecular catalysts – molecules which, like enzymes, can trigger or accelerate certain chemical reactions – function, and what effects do they have? A team of chemists at the University of Oldenburg has come closer to the answers using a model molecule that functions like a molecular nanobattery. It consists of several titanium centres linked to each other by a single layer of interconnected carbon and nitrogen atoms. The seven-member research team recently published its findings, which combine the results of three multi-year PhD research projects, in “ChemPhysChem”. The physical chemistry and chemical physics journal featured the basic research from Oldenburg on its cover.
A molecule like a nanobattery: Chemical scientists decipher complex electronic structure of a three-nuclear metallorganic compound with the capacity of donating and receiving multiple electrons
Oldenburg, Germany | Posted on December 8th, 2020
To gain a better understanding of how the molecule works, the researchers, headed by first authors Dr. Aleksandra Markovic and Luca Gerhards and corresponding author Prof. Dr. Gunther Wittstock, performed electrochemical and spectroscopic experiments and used the university’s high-performance computing cluster for their calculations. Wittstock sees the publication of the paper as a “success story” for both the Research Training Groups within which the PhD projects were conducted and for the university’s computing cluster. “Without the high-performance computing infrastructure, we would not have been able to perform the extensive calculations required to decipher the behaviour of the molecule,” says Wittstock. “This underlines the importance of such computing clusters for current research.”
In the paper, the authors present the results of their analysis of a molecular structure, the prototype for which was the result of an unexpected chemical reaction first reported by the University of Oldenburg’s Chemistry Department in 2006. It is a highly complex molecular structure in which three titanium centres (commonly referred to in high school lessons as titanium ions) are connected to each other by a bridging ligand consisting of carbon and nitrogen. Such a compound would be expected to be able to accept and release several electrons through the exchange of electrons between the metal centres among other reasons.
Gaining a proper understanding of these processes is of particular interest not only for basic research, but also for triggering or accelerating important reactions in which more than one electron is transferred. Such reactions remain a major challenge in technical systems, for which there is still no satisfactory solution. “Many efforts are currently focused on this objective,” says Wittstock. One example is fuel cell technology, which requires the simultaneous transfer of four electrons to one oxygen molecule in order to achieve a flow of electrons from hydrogen to oxygen, he explains. “Such multi-electron reactions also have great potential for saving materials or energy in chemical production.”
The model molecular compound consisting of the bridging ligand and the titanium centres was specifically designed to help the scientists gain a detailed understanding of how compounds with several metal centres are able to accept and release electrons. The scientists excited the molecule by light, to which the molecules responded differently depending on the number of accepted and released electrons. Unfortunately, the molecule made in 2006 proved to be poorly soluble in most solvents and therefore difficult to study. Using chemical synthesis, Dr. Pia Sander, a co-author of the paper, added propeller-like molecular motifs to the compound to improve its solubility. This provided the basis for Markovic’s experiments, which revealed that the model compound could accept a total of three electrons or release six electrons – an unusually high capacity for a single molecule. In each of these reactions, not only the visible colour of the molecule changes, but the absorption of light in the spectral ranges which are invisible to the human eye. Initially, however, the precise changes in the molecule with different numbers of electrons could not be determined on the basis of those spectral ranges.
This is where Luca Gerhards and the university’s computing cluster came into play. Although common explanations are based on the premise that in each transition excited by light only the energy of a single electron changes, co-author Gerhards avoided these simplifying assumptions in his quantum chemical equations. This made the calculations even more complex and kept the high-performance computing cluster busy for months. In the end, the result came as a surprise to everyone involved: Several electrons change their energy levels simultaneously when light hits the molecule under study. Moreover, this charge is not stored in the titanium, as would be expected, but mainly in the bridging ligand, the “link” between the titanium centres.
As Wittstock explains, the metal centres thus provide a positively charged “frame” for electron storage, as in a “nanobattery”. The model molecule – and by extension a whole class of similar compounds – has turned out to be a “mini segment of an energy storage material”. Although their full potential cannot be determined at this stage, Wittstock believes that such “frames” with molecular charge storage motifs could become a new design element in complex molecular catalysts for multi-electron reactions.
###
The PhD research projects were conducted in research groups led by the Oldenburg chemistry professors Wittstock, Prof. Dr. Rüdiger Beckhaus and Prof. Dr. Thorsten Klüner. The research of the first two projects was interlinked within the framework of the concluded “Nano Energy” Research Training Group, which was funded by the Ministry for Science and Culture of the State of Lower Saxony. The third project involving quantum chemical calculations is part of the Research Training Group “Chemical Bond Activation”, which the German Research Foundation (DFG) has been funding since 2017.
####
For more information, please click here
Contacts:
Prof. Dr Gunther Wittstock
Copyright © University of Oldenburg
If you have a comment, please Contact us.
Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
News and information
![]() Faraday fabrics? MXene-coated fabric could contain electronic interference in wearable devices December 11th, 2020
Faraday fabrics? MXene-coated fabric could contain electronic interference in wearable devices December 11th, 2020
![]() Stretchable micro-supercapacitors to self-power wearable devices December 11th, 2020
Stretchable micro-supercapacitors to self-power wearable devices December 11th, 2020
![]() In new step toward quantum tech, scientists synthesize ‘bright’ quantum bits: Innovative step by Northwestern, UChicago scientists could boost computing, sensing December 10th, 2020
In new step toward quantum tech, scientists synthesize ‘bright’ quantum bits: Innovative step by Northwestern, UChicago scientists could boost computing, sensing December 10th, 2020
Chemistry
![]() RUDN University physicists described a new type of amorphous solid bodies December 4th, 2020
RUDN University physicists described a new type of amorphous solid bodies December 4th, 2020
![]() Having it Both Ways: A Combined Strategy in Catalyst Design for Suzuki Cross-Couplings December 2nd, 2020
Having it Both Ways: A Combined Strategy in Catalyst Design for Suzuki Cross-Couplings December 2nd, 2020
![]() Light-controlled nanomachine controls catalysis: A molecular motor enables the speed of chemical processes to be controlled using light impulses November 23rd, 2020
Light-controlled nanomachine controls catalysis: A molecular motor enables the speed of chemical processes to be controlled using light impulses November 23rd, 2020
![]() Smaller than EverExploring the Unusual Properties of Quantum-sized Materials November 13th, 2020
Smaller than EverExploring the Unusual Properties of Quantum-sized Materials November 13th, 2020
Possible Futures
![]() Faraday fabrics? MXene-coated fabric could contain electronic interference in wearable devices December 11th, 2020
Faraday fabrics? MXene-coated fabric could contain electronic interference in wearable devices December 11th, 2020
![]() Stretchable micro-supercapacitors to self-power wearable devices December 11th, 2020
Stretchable micro-supercapacitors to self-power wearable devices December 11th, 2020
![]() In new step toward quantum tech, scientists synthesize ‘bright’ quantum bits: Innovative step by Northwestern, UChicago scientists could boost computing, sensing December 10th, 2020
In new step toward quantum tech, scientists synthesize ‘bright’ quantum bits: Innovative step by Northwestern, UChicago scientists could boost computing, sensing December 10th, 2020
Discoveries
![]() Faraday fabrics? MXene-coated fabric could contain electronic interference in wearable devices December 11th, 2020
Faraday fabrics? MXene-coated fabric could contain electronic interference in wearable devices December 11th, 2020
![]() Stretchable micro-supercapacitors to self-power wearable devices December 11th, 2020
Stretchable micro-supercapacitors to self-power wearable devices December 11th, 2020
![]() In new step toward quantum tech, scientists synthesize ‘bright’ quantum bits: Innovative step by Northwestern, UChicago scientists could boost computing, sensing December 10th, 2020
In new step toward quantum tech, scientists synthesize ‘bright’ quantum bits: Innovative step by Northwestern, UChicago scientists could boost computing, sensing December 10th, 2020
Announcements
![]() Faraday fabrics? MXene-coated fabric could contain electronic interference in wearable devices December 11th, 2020
Faraday fabrics? MXene-coated fabric could contain electronic interference in wearable devices December 11th, 2020
![]() Stretchable micro-supercapacitors to self-power wearable devices December 11th, 2020
Stretchable micro-supercapacitors to self-power wearable devices December 11th, 2020
![]() In new step toward quantum tech, scientists synthesize ‘bright’ quantum bits: Innovative step by Northwestern, UChicago scientists could boost computing, sensing December 10th, 2020
In new step toward quantum tech, scientists synthesize ‘bright’ quantum bits: Innovative step by Northwestern, UChicago scientists could boost computing, sensing December 10th, 2020
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Faraday fabrics? MXene-coated fabric could contain electronic interference in wearable devices December 11th, 2020
Faraday fabrics? MXene-coated fabric could contain electronic interference in wearable devices December 11th, 2020
![]() Stretchable micro-supercapacitors to self-power wearable devices December 11th, 2020
Stretchable micro-supercapacitors to self-power wearable devices December 11th, 2020
![]() In new step toward quantum tech, scientists synthesize ‘bright’ quantum bits: Innovative step by Northwestern, UChicago scientists could boost computing, sensing December 10th, 2020
In new step toward quantum tech, scientists synthesize ‘bright’ quantum bits: Innovative step by Northwestern, UChicago scientists could boost computing, sensing December 10th, 2020
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() Stretchable micro-supercapacitors to self-power wearable devices December 11th, 2020
Stretchable micro-supercapacitors to self-power wearable devices December 11th, 2020
![]() Multiple Semiconductor Type Switching To Boost Thermoelectric Conversion of Waste Heat December 9th, 2020
Multiple Semiconductor Type Switching To Boost Thermoelectric Conversion of Waste Heat December 9th, 2020
![]() Staying ahead of the curve with 3D curved graphene November 20th, 2020
Staying ahead of the curve with 3D curved graphene November 20th, 2020
![]() Improving quantum dot interactions, one layer at a time: Scientists have found a way to control an interaction between quantum dots that could lead to more efficient solar cells November 20th, 2020
Improving quantum dot interactions, one layer at a time: Scientists have found a way to control an interaction between quantum dots that could lead to more efficient solar cells November 20th, 2020










