Home > Press > Copper doping enables safer, cost-effective hydrogen peroxide production
 |
| To produce safer, more economic and environmental hydrogen peroxide, an international research team turned to copper.
CREDIT Nano Research |
Abstract:
Hydrogen peroxide, the common household antiseptic used to clean cuts and scrapes, can also power space shuttles. While the version sold in pharmacies is far less concentrated than what is used in industry, the mere reduction of two hydrogen and two oxygen atoms into water and an extra oxygen can produce big results. The compounds production is costly, though, requiring expensive metals to trigger the necessary chemical reactions that, when left unchecked, can produce unintentional explosions.
Copper doping enables safer, cost-effective hydrogen peroxide production
Beijing, China | Posted on February 11th, 2022
To produce safer, more economic and environmental hydrogen peroxide, an international research team turned to copper. The common metal helped reduce the number of manufacturing steps, making the resulting hydrogen peroxide more stable, efficient and cost-effective. They published their work on Jan. 11 in Nano Research.
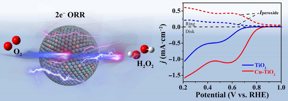
Hydrogen peroxide is considered a high-value oxidant a substance that can accept electrons from other substances, according to paper author Qian Liu, associate professor at Chengdu Universitys Institute for Advanced Study. It is traditionally produced through a multi-step process in which an expensive metal, such as palladium, electrochemically reacts with a chemical compound containing hydrogen and oxygen to reduce the oxygens electrons by four to produce hydrogen peroxide and unwanted organic waste.
The four-electron reduction process generates hydrogen peroxide and water there is competition between the two processes, Liu said. As such, in the process of designing and preparing catalysts, we need to satisfy a two-electron reaction process to selectively produce hydrogen peroxide as much as possible to reduce unnecessary energy loss.
The researchers opted to use titanium dioxide as an abundant, non-toxic and stable catalyst, but need to enhance it to achieve a two-electron reaction process.
We doped the titanium dioxide with copper to naturally increase oxygen vacancy concentration, leading to improved electronic conductivity and better generation of hydrogen peroxide, said paper author Shihai Yan, associate professor, College of Chemistry and Pharmaceutical Sciences, Qingdao Agricultural University.
Copper serves as a heteroatom, which allows the researchers to manipulate the electronic structure of titanium dioxide. This enhanced catalyst can then create new atomic vacancies in the reduction compounds, encouraging one product over another. For example, when electrochemically reducing molecular hydrogen and oxygen, the addition of copper helps create more spots for oxygen to bond with hydrogen to produce hydrogen peroxide. Instead of a competition for the constituents to become water or hydrogen peroxide, the latter gets a boost, while the rest burns off as gas. When the process is contained in liquid, thats a relatively harmless side effect.
Two-electron electroreduction of oxygen into hydrogen peroxide in an aqueous environment provides a safe, sustainable and energy-saving method for on-demand production, said Xuping Sun, professor, Institute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China
Copper-doped titanium dioxide exhibits a significantly improved selectivity of up to 91.2% for hydrogen peroxide, meaning that most of components reduce into the desired product. Moreover, it also shows a larger yield and good stability.
Next, the researchers plan to design and synthesize copper-doped titanium dioxide catalysts against practical requirements to achieve large-scale industrial production.
This study provides a new route to adjust the electronic structure of metal oxide by heteroatom doping as high efficiency electrocatalysts for oxygen reduction reaction to produce hydrogen peroxide, Liu said.
Other contributors include co-first author Zhiqin Deng, co-first author Li Li, Yuchun Ren, Je Liang, Kai Dong and Tingshuai Li, Institute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China; Chaoqun Ma, College of Chemistry and Pharmaceutical Sciences, Qingdao Agricultural University; Yonglan Luo, Institute for Advanced Study, Chengdu University; Bo Tang, College of Chemistry, Chemical Engineering and Materials Science, Shandong Normal University; Yang Liu and Shuyan Gao, School of Materials Science and Engineering, Henan Normal University; and Abdullah M. Asin, Chemistry Department, Faculty of Science & Center of Excellence for Advanced Materials Research, King Abdulaziz University.
The National Natural Science Foundation of China supported this work.
####
About Tsinghua University Press
Established in 1980, belonging to Tsinghua University, Tsinghua University Press (TUP) is a leading comprehensive higher education and professional publisher in China. Committed to building a top-level global cultural brand, after 41 years of development, TUP has established an outstanding managerial system and enterprise structure, and delivered multimedia and multi-dimensional publications covering books, audio, video, electronic products, journals and digital publications. In addition, TUP actively carries out its strategic transformation from educational publishing to content development and service for teaching & learning and was named First-class National Publisher for achieving remarkable results.
About Nano Research
Nano Research is a peer-reviewed, international and interdisciplinary research journal, sponsored by Tsinghua University and the Chinese Chemical Society. It offers readers an attractive mix of authoritative and comprehensive reviews and original cutting-edge research papers. After more than 10 years of development, it has become one of the most influential academic journals in the nano field. Rapid review to ensure quick publication is a key feature of Nano Research. In 2020 InCites Journal Citation Reports, Nano Research has an Impact Factor of 8.897 (8.696, 5 years), the total cites reached 23150, and the number of highly cited papers reached 129, ranked among the top 2.5% of over 9000 academic journals, ranking first in China’s international academic journals.
For more information, please click here
Contacts:
Yao Meng
Tsinghua University Press
Office: 86-108-347-0574
Copyright © Tsinghua University Press
If you have a comment, please Contact us.
Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
News and information
![]()
NGI advances graphene spintronics as 1D contacts improve mobility in nano-scale devices February 11th, 2022
![]()
University of Oklahoma scientists quantum technology work garners international attention February 11th, 2022
![]()
Reusable Catalyst Makes CH Bond Oxidation Using Oxygen Easier and More Efficient February 11th, 2022
![]()
Wise-integration and EDOM Technology Announce Channel Partnership for GaN IC Power Semiconductor Deployment February 11th, 2022
![]()
Polymer fibers with graphene nanotubes make it possible to heat hard-to-reach, complex-shaped items February 11th, 2022
Chemistry
![]()
Reusable Catalyst Makes CH Bond Oxidation Using Oxygen Easier and More Efficient February 11th, 2022
Govt.-Legislation/Regulation/Funding/Policy
![]()
Scientists use DNA to assemble complex nanomaterials: Researchers create DNA nano-chambers with bonds that can control the assembly of targeted nanoparticle structures February 11th, 2022
![]()
Acceleration of cancer biomarker detection for point of care diagnostics January 28th, 2022
![]()
Shining a light on synthetic dimensions January 28th, 2022
Possible Futures
![]()
Scientists use DNA to assemble complex nanomaterials: Researchers create DNA nano-chambers with bonds that can control the assembly of targeted nanoparticle structures February 11th, 2022
![]()
NGI advances graphene spintronics as 1D contacts improve mobility in nano-scale devices February 11th, 2022
![]()
Reusable Catalyst Makes CH Bond Oxidation Using Oxygen Easier and More Efficient February 11th, 2022
Materials/Metamaterials
![]()
Eyebrow-raising: Researchers reveal why nanowires stick to each other February 11th, 2022
![]()
Nanotube fibers stand strong — but for how long? Rice scientists calculate how carbon nanotubes and their fibers experience fatigue December 24th, 2021
![]()
Nanodiamonds are key to efficient hydrogen purification: Nanodiamonds may be tiny, but they can help with one of the biggest problems facing humanity today: Climate change December 17th, 2021
![]()
Texas A&M chemist recognized for paving the way toward artificial intelligence and energy conversion December 10th, 2021
Announcements
![]()
University of Oklahoma scientists quantum technology work garners international attention February 11th, 2022
![]()
Reusable Catalyst Makes CH Bond Oxidation Using Oxygen Easier and More Efficient February 11th, 2022
![]()
Wise-integration and EDOM Technology Announce Channel Partnership for GaN IC Power Semiconductor Deployment February 11th, 2022
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]()
Eyebrow-raising: Researchers reveal why nanowires stick to each other February 11th, 2022
![]()
Quantum tech in space? Scientists design remote monitoring system for inaccessible quantum devices February 11th, 2022
![]()
NGI advances graphene spintronics as 1D contacts improve mobility in nano-scale devices February 11th, 2022
![]()
University of Oklahoma scientists quantum technology work garners international attention February 11th, 2022
Aerospace/Space
![]()
Quantum tech in space? Scientists design remote monitoring system for inaccessible quantum devices February 11th, 2022
![]()
Polymer fibers with graphene nanotubes make it possible to heat hard-to-reach, complex-shaped items February 11th, 2022
![]()
Super-resolved imaging of a single cold atom on a nanosecond timescale January 7th, 2022
![]()
Researchers develop polyimide-mica nanocomposite film with high resistance to low earth orbit environments December 3rd, 2021










