Home > Press > Go with the flow: Scientists design new grid batteries for renewable energy: New blueprint for affordable, sustainable ‘flow batteries’ developed at Berkeley Lab could accelerate an electrical grid powered by the sun and wind
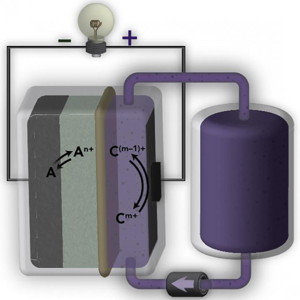 |
| Schematic of a flow battery with an ion-selective AquaPIM membrane (noted in beige). Berkeley Lab scientists discovered that such a model could predict the lifetime and efficiency of a flow battery for the electric grid without having to build an entire device.
CREDIT Brett Helms/Berkeley Lab |
Abstract:
How do you store renewable energy so it’s there when you need it, even when the sun isn’t shining or the wind isn’t blowing? Giant batteries designed for the electrical grid – called flow batteries, which store electricity in tanks of liquid electrolyte – could be the answer, but so far utilities have yet to find a cost-effective battery that can reliably power thousands of homes throughout a lifecycle of 10 to 20 years.
Go with the flow: Scientists design new grid batteries for renewable energy: New blueprint for affordable, sustainable ‘flow batteries’ developed at Berkeley Lab could accelerate an electrical grid powered by the sun and wind
Berkeley, CA | Posted on November 8th, 2019
Now, a battery membrane technology developed by researchers at the U.S. Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) may point to a solution.
As reported in the journal of Joule, the researchers developed a versatile yet affordable battery membrane – from a class of polymers known as AquaPIMs. This class of polymers makes long-lasting and low-cost grid batteries possible based solely on readily available materials such as zinc, iron, and water. The team also developed a simple model showing how different battery membranes impact the lifetime of the battery, which is expected to accelerate early stage R&D for flow-battery technologies, particularly in the search for a suitable membrane for different battery chemistries.
“Our AquaPIM membrane technology is well-positioned to accelerate the path to market for flow batteries that use scalable, low-cost, water-based chemistries,” said Brett Helms, a principal investigator in the Joint Center for Energy Storage Research (JCESR) and staff scientist at Berkeley Lab’s Molecular Foundry who led the study. “By using our technology and accompanying empirical models for battery performance and lifetime, other researchers will be able to quickly evaluate the readiness of each component that goes into the battery, from the membrane to the charge-storing materials. This should save time and resources for researchers and product developers alike.”
Most grid battery chemistries have highly alkaline (or basic) electrodes – a positively charged cathode on one side, and a negatively charged anode on the other side. But current state-of-the-art membranes are designed for acidic chemistries, such as the fluorinated membranes found in fuel cells, but not for alkaline flow batteries. (In chemistry, pH is a measure of the hydrogen ion concentration of a solution. Pure water has a pH of 7 and is considered neutral. Acidic solutions have a high concentration of hydrogen ions, and are described as having a low pH, or a pH below 7. On the other hand, alkaline solutions have low concentrations of hydrogen ions and therefore have a high pH, or a pH above 7. In alkaline batteries, the pH can be as high as 14 or 15.)
Fluorinated polymer membranes are also expensive. According to Helms, they can make up 15% to 20% of the battery’s cost, which can run in the range of $300/kWh.
One way to drive down the cost of flow batteries is to eliminate the fluorinated polymer membranes altogether and come up with a high-performing yet cheaper alternative such as AquaPIMs, said Miranda Baran, a graduate student researcher in Helms’ research group and the study’s lead author. Baran is also a Ph.D. student in the Department of Chemistry at UC Berkeley.
Getting back to basics
Helms and co-authors discovered the AquaPIM technology – which stands for “aqueous-compatible polymers of intrinsic microporosity” – while developing polymer membranes for aqueous alkaline (or basic) systems as part of a collaboration with co-author Yet-Ming Chiang, a principal investigator in JCESR and Kyocera Professor of Materials Science and Engineering at the Massachusetts Institute of Technology (MIT).
Through these early experiments, the researchers learned that membranes modified with an exotic chemical called an “amidoxime” allowed ions to quickly travel between the anode and cathode.
Later, while evaluating AquaPIM membrane performance and compatibility with different grid battery chemistries – for example, one experimental setup used zinc as the anode and an iron-based compound as the cathode – the researchers discovered that AquaPIM membranes lead to remarkably stable alkaline cells.
In addition, they found that the AquaPIM prototypes retained the integrity of the charge-storing materials in the cathode as well as in the anode. When the researchers characterized the membranes at Berkeley Lab’s Advanced Light Source (ALS), the researchers found that these characteristics were universal across AquaPIM variants.
Baran and her collaborators then tested how an AquaPIM membrane would perform with an aqueous alkaline electrolyte. In this experiment, they discovered that under alkaline conditions, polymer-bound amidoximes are stable – a surprising result considering that organic materials are not typically stable at high pH.
Such stability prevented the AquaPIM membrane pores from collapsing, thus allowing them to stay conductive without any loss in performance over time, whereas the pores of a commercial fluoro-polymer membrane collapsed as expected, to the detriment of its ion transport properties, Helms explained.
This behavior was further corroborated with theoretical studies by Artem Baskin, a postdoctoral researcher working with David Prendergast, who is the acting director of Berkeley Lab’s Molecular Foundry and a principal investigator in JCESR along with Chiang and Helms.
Baskin simulated structures of AquaPIM membranes using computational resources at Berkeley Lab’s National Energy Research Scientific Computing Center (NERSC) and found that the structure of the polymers making up the membrane were significantly resistant to pore collapse under highly basic conditions in alkaline electrolytes.
A screen test for better batteries
While evaluating AquaPIM membrane performance and compatibility with different grid battery chemistries, the researchers developed a model that tied the performance of the battery to the performance of various membranes. This model could predict the lifetime and efficiency of a flow battery without having to build an entire device. They also showed that similar models could be applied to other battery chemistries and their membranes.
“Typically, you’d have to wait weeks if not months to figure out how long a battery will last after assembling the entire cell. By using a simple and quick membrane screen, you could cut that down to a few hours or days,” Helms said.
The researchers next plan to apply AquaPIM membranes across a broader scope of aqueous flow battery chemistries, from metals and inorganics to organics and polymers. They also anticipate that these membranes are compatible with other aqueous alkaline zinc batteries, including batteries that use either oxygen, manganese oxide, or metal-organic frameworks as the cathode.
Researchers from Berkeley Lab, UC Berkeley, Massachusetts Institute of Technology, and Istituto Italiano di Tecnologia participated in the study.
###
This work was supported by the Joint Center for Energy Storage Research (JCESR), an Energy Innovation Hub funded by the U.S. Department of Energy, Office of Science. Additional funding was provided by the Center for Gas Separations Relevant to Clean Energy Technologies, a DOE Office of Science Energy Frontier Research Center.
Portions of the work, including polymer synthesis and characterization, were carried out at Berkeley Lab’s Molecular Foundry, a DOE Office of Science User Facility that specializes in nanoscale science.
The study also used GIWAXS (grazing-incidence wide angle X-ray scattering) instruments at the ALS to characterize the AquaPIMs, and supercomputing resources at NERSC to model the polymer. The ALS and NERSC are DOE Office of Science User Facilities.
This technology is available for licensing and collaboration. If interested, please contact Berkeley Lab’s Intellectual Property Office, .
####
About Lawrence Berkeley National Laboratory
Founded in 1931 on the belief that the biggest scientific challenges are best addressed by teams, Lawrence Berkeley National Laboratory and its scientists have been recognized with 13 Nobel Prizes. Today, Berkeley Lab researchers develop sustainable energy and environmental solutions, create useful new materials, advance the frontiers of computing, and probe the mysteries of life, matter, and the universe. Scientists from around the world rely on the Lab’s facilities for their own discovery science. Berkeley Lab is a multiprogram national laboratory, managed by the University of California for the U.S. Department of Energy’s Office of Science.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit energy.gov/science.
For more information, please click here
Contacts:
Theresa Duque
510-495-2418
@BerkeleyLab
Copyright © Lawrence Berkeley National Laboratory
If you have a comment, please Contact us.
Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
News and information
![]() Arrowhead Pharmaceuticals to Webcast Fiscal 2019 Year End Results November 11th, 2019
Arrowhead Pharmaceuticals to Webcast Fiscal 2019 Year End Results November 11th, 2019
![]() Scientists probe the limits of ice: Transition between ice and liquid water gets fuzzy at the nanoscale November 9th, 2019
Scientists probe the limits of ice: Transition between ice and liquid water gets fuzzy at the nanoscale November 9th, 2019
![]() Arrowhead and Collaborator Janssen Present Phase 2 Clinical Data for Investigational Hepatitis B Regimens at The Liver Meeting® 2019 November 8th, 2019
Arrowhead and Collaborator Janssen Present Phase 2 Clinical Data for Investigational Hepatitis B Regimens at The Liver Meeting® 2019 November 8th, 2019
Laboratories
![]() Argonne collaborates to review current battery recycling processes for electric vehicles November 8th, 2019
Argonne collaborates to review current battery recycling processes for electric vehicles November 8th, 2019
![]() Shedding new light on the charging of lithium-ion batteries November 1st, 2019
Shedding new light on the charging of lithium-ion batteries November 1st, 2019
![]() New drug-delivery technology promises efficient, targeted cancer treatment October 22nd, 2019
New drug-delivery technology promises efficient, targeted cancer treatment October 22nd, 2019
Govt.-Legislation/Regulation/Funding/Policy
![]() Scientists probe the limits of ice: Transition between ice and liquid water gets fuzzy at the nanoscale November 9th, 2019
Scientists probe the limits of ice: Transition between ice and liquid water gets fuzzy at the nanoscale November 9th, 2019
![]() Argonne collaborates to review current battery recycling processes for electric vehicles November 8th, 2019
Argonne collaborates to review current battery recycling processes for electric vehicles November 8th, 2019
![]() Nanoparticle orientation offers a way to enhance drug delivery: Coating particles with ‘right-handed’ molecules could help them penetrate cancer cells more easily November 5th, 2019
Nanoparticle orientation offers a way to enhance drug delivery: Coating particles with ‘right-handed’ molecules could help them penetrate cancer cells more easily November 5th, 2019
Possible Futures
![]() Arrowhead Pharmaceuticals to Webcast Fiscal 2019 Year End Results November 11th, 2019
Arrowhead Pharmaceuticals to Webcast Fiscal 2019 Year End Results November 11th, 2019
![]() Scientists probe the limits of ice: Transition between ice and liquid water gets fuzzy at the nanoscale November 9th, 2019
Scientists probe the limits of ice: Transition between ice and liquid water gets fuzzy at the nanoscale November 9th, 2019
![]() Self-assembled microspheres of silica to cool surfaces without energy consumption November 8th, 2019
Self-assembled microspheres of silica to cool surfaces without energy consumption November 8th, 2019
Discoveries
![]() Scientists probe the limits of ice: Transition between ice and liquid water gets fuzzy at the nanoscale November 9th, 2019
Scientists probe the limits of ice: Transition between ice and liquid water gets fuzzy at the nanoscale November 9th, 2019
![]() Thorium superconductivity: Scientists discover a new high-temperature superconductor November 8th, 2019
Thorium superconductivity: Scientists discover a new high-temperature superconductor November 8th, 2019
![]() Self-assembled microspheres of silica to cool surfaces without energy consumption November 8th, 2019
Self-assembled microspheres of silica to cool surfaces without energy consumption November 8th, 2019
Announcements
![]() Arrowhead Pharmaceuticals to Webcast Fiscal 2019 Year End Results November 11th, 2019
Arrowhead Pharmaceuticals to Webcast Fiscal 2019 Year End Results November 11th, 2019
![]() Scientists probe the limits of ice: Transition between ice and liquid water gets fuzzy at the nanoscale November 9th, 2019
Scientists probe the limits of ice: Transition between ice and liquid water gets fuzzy at the nanoscale November 9th, 2019
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Scientists probe the limits of ice: Transition between ice and liquid water gets fuzzy at the nanoscale November 9th, 2019
Scientists probe the limits of ice: Transition between ice and liquid water gets fuzzy at the nanoscale November 9th, 2019
![]() Self-assembled microspheres of silica to cool surfaces without energy consumption November 8th, 2019
Self-assembled microspheres of silica to cool surfaces without energy consumption November 8th, 2019
![]() Arrowhead and Collaborator Janssen Present Phase 2 Clinical Data for Investigational Hepatitis B Regimens at The Liver Meeting® 2019 November 8th, 2019
Arrowhead and Collaborator Janssen Present Phase 2 Clinical Data for Investigational Hepatitis B Regimens at The Liver Meeting® 2019 November 8th, 2019
Tools
![]() Picosun expands selection of biocompatible ALD materials for medical applications November 4th, 2019
Picosun expands selection of biocompatible ALD materials for medical applications November 4th, 2019
![]() Extracting hidden quantum information from a light source October 25th, 2019
Extracting hidden quantum information from a light source October 25th, 2019
![]() Pinpointing biomolecules with nanometer accuracy October 21st, 2019
Pinpointing biomolecules with nanometer accuracy October 21st, 2019
Patents/IP/Tech Transfer/Licensing
![]() Self-assembled microspheres of silica to cool surfaces without energy consumption November 8th, 2019
Self-assembled microspheres of silica to cool surfaces without energy consumption November 8th, 2019
![]() New drug-delivery technology promises efficient, targeted cancer treatment October 22nd, 2019
New drug-delivery technology promises efficient, targeted cancer treatment October 22nd, 2019
![]() Highest-throughput 3D printer is future of manufacturing: Rapid manufacturing on-demand could put warehouses, molds into the past October 17th, 2019
Highest-throughput 3D printer is future of manufacturing: Rapid manufacturing on-demand could put warehouses, molds into the past October 17th, 2019
![]() New materials to help stop lithium-ion battery fires, explosions and improve battery performance October 2nd, 2019
New materials to help stop lithium-ion battery fires, explosions and improve battery performance October 2nd, 2019
Energy
![]() Cage molecules act as molecular sieves for hydrogen isotope separation November 1st, 2019
Cage molecules act as molecular sieves for hydrogen isotope separation November 1st, 2019
![]() New technique lets researchers map strain in next-gen solar cells November 1st, 2019
New technique lets researchers map strain in next-gen solar cells November 1st, 2019
![]() Promising discovery could lead to a better, cheaper solar cell: Scientific instrument made at McGill reveals liquid-like properties of a solid substance November 1st, 2019
Promising discovery could lead to a better, cheaper solar cell: Scientific instrument made at McGill reveals liquid-like properties of a solid substance November 1st, 2019
![]() How perovskite in solar cells recrystallizes and why modified carbon nanotubes can help overcome the reproducibility problem by making use of this October 18th, 2019
How perovskite in solar cells recrystallizes and why modified carbon nanotubes can help overcome the reproducibility problem by making use of this October 18th, 2019
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() Argonne collaborates to review current battery recycling processes for electric vehicles November 8th, 2019
Argonne collaborates to review current battery recycling processes for electric vehicles November 8th, 2019
![]() Self-assembled microspheres of silica to cool surfaces without energy consumption November 8th, 2019
Self-assembled microspheres of silica to cool surfaces without energy consumption November 8th, 2019
![]() Shedding new light on the charging of lithium-ion batteries November 1st, 2019
Shedding new light on the charging of lithium-ion batteries November 1st, 2019
Solar/Photovoltaic
![]() Self-assembled microspheres of silica to cool surfaces without energy consumption November 8th, 2019
Self-assembled microspheres of silica to cool surfaces without energy consumption November 8th, 2019
![]() New technique lets researchers map strain in next-gen solar cells November 1st, 2019
New technique lets researchers map strain in next-gen solar cells November 1st, 2019
![]() Promising discovery could lead to a better, cheaper solar cell: Scientific instrument made at McGill reveals liquid-like properties of a solid substance November 1st, 2019
Promising discovery could lead to a better, cheaper solar cell: Scientific instrument made at McGill reveals liquid-like properties of a solid substance November 1st, 2019
![]() How perovskite in solar cells recrystallizes and why modified carbon nanotubes can help overcome the reproducibility problem by making use of this October 18th, 2019
How perovskite in solar cells recrystallizes and why modified carbon nanotubes can help overcome the reproducibility problem by making use of this October 18th, 2019










