Home > Press > Previously unknown pathway to batteries with high energy, low cost and long life: Newly discovered reaction mechanism overcomes rapid performance decline in lithium-sulfur batteries
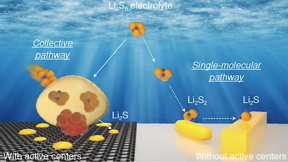 |
| Different reaction pathways from lithium polysulfide (Li₂S₆) to lithium sulfide (Li₂S) in lithium-sulfur batteries with (left) and without (right) catalyst in sulfur cathode.
CREDIT (Image by Argonne National Laboratory.) |
Abstract:
Scientists discover surprising pathway to better lithium-sulfur batteries by visualizing reactions at the atomic scale.
Previously unknown pathway to batteries with high energy, low cost and long life: Newly discovered reaction mechanism overcomes rapid performance decline in lithium-sulfur batteries
Lemont, IL | Posted on September 8th, 2023
The road from breakthrough in the lab to practical technology can be a long and bumpy one. The lithium-sulfur battery is an example. It has notable advantages over current lithium-ion batteries powering vehicles. But it has yet to dent the market despite intense development over many years.
That situation could change in the future thanks to the efforts of scientists at the U.S. Department of Energys (DOE) Argonne National Laboratory. Over the past decade, they have made several pivotal discoveries related to lithium-sulfur batteries. Their latest revelation, published in Nature, unlocks a previously unknown reaction mechanism that addresses a major shortcoming the batteries very short lifetimes.
Gui-Liang Xu, chemist in Argonnes Chemical Sciences and Engineering division, stated that Our teams efforts could bring the U.S. one large step closer to a greener and more sustainable transportation landscape.
Lithium-sulfur batteries offer three significant advantages over current lithium-ion batteries. Firstly, they can store two to three times more energy in a given volume, resulting in longer vehicle ranges. Secondly, their lower cost, facilitated by the abundance and affordability of sulfur, makes them economically viable. Lastly, these batteries do not rely on critical resources like cobalt and nickel, which may face shortages in the future.
Despite these benefits, transitioning from laboratory success to commercial viability has proven elusive. Laboratory cells have shown promising results, but when scaled up to commercial size, their performance rapidly declines with repeated charge and discharge.
The underlying cause of this performance decline lies in the dissolution of sulfur from the cathode during discharge, leading to the formation of soluble lithium polysulfides (Li2S6). These compounds flow into the lithium metal negative electrode (anode) during charging, further exacerbating the issue. Consequently, the loss of sulfur from the cathode and alterations in the anode composition significantly hinder the batterys performance during cycling.
In a recent earlier study, Argonne scientists developed a catalytic material that, when added in a small amount to the sulfur cathode, essentially eliminated the sulfur loss problem. While this catalyst showed promise in both laboratory and commercial-size cells, its atomic-scale working mechanism remained an enigma until now.
The teams most recent research shed light on this mechanism. In the absence of the catalyst, lithium polysulfides form at the cathode surface and undergo a series of reactions, ultimately converting the cathode to lithium sulfide (Li2S).
But the presence of a small amount of catalyst in the cathode makes all the difference, Xu said. A much different reaction pathway follows, one free of intermediate reaction steps.
Key is the formation of dense nanoscale bubbles of lithium polysulfides on the cathode surface, which do not appear without the catalyst. These lithium polysulfides rapidly spread throughout the cathode structure during discharge and transform to lithium sulfide consisting of nanoscale crystallites. This process prevents the sulfur loss and performance decline in commercial-size cells.
In unlocking this black box around the reaction mechanism, the scientists employed cutting-edge characterization techniques. Analyses of the catalysts structure with the intense synchrotron X-ray beams at beamline 20-BM of the Advanced Photon Source, a DOE Office of Science user facility, revealed that it plays a critical role in the reaction pathway. The catalyst structure affects the shape and composition of the final product upon discharge, as well as the intermediate products. With the catalyst, nanocrystalline lithium sulfide forms upon full discharge. Without the catalyst, microscale rod-shaped structures form instead.
Our teams efforts could bring the U.S. one large step closer to a greener and more sustainable transportation landscape. Gui-Liang Xu, chemist in Argonnes Chemical Sciences and Engineering division
Another vital technique, developed at Xiamen University, allowed the team to visualize the electrode-electrolyte interface at the nanoscale while a test cell was working. This newly invented technique helped connect changes at the nanoscale to the behavior of an operating cell.
Based on our exciting discovery, we will be doing more research to design even better sulfur cathodes, Xu noted. It would also be worthwhile to explore whether this mechanism applies to other next-generation batteries, such as sodium-sulfur.
With this the teams latest breakthrough, the future of lithium-sulfur batteries appears brighter, offering a more sustainable and eco-friendly solution for the transportation industry.
An article on this research appeared in Nature. In addition to Xu, authors include Shiyuan Zhou, Jie Shi, Sangui Liu, Gen Li, Fei Pei, Youhu Chen, Junxian Deng, Qizheng Zheng, Jiayi Li, Chen Zhao, Inhui Hwang, Cheng-Jun Sun, Yuzi Liu, Yu Deng, Ling Huang, Yu Qiao, Jian-Feng Chen, Khalil Amine, Shi-Gang Sun and Hong-Gang Liao.
Other participating institutions include Xiamen University, Beijing University of Chemical Technology and Nanjing University. The Argonne research was supported by the DOE Office of Vehicle Technologies in the Office of Energy Efficiency and Renewable Energy.
About the Advanced Photon Source
The U. S. Department of Energy Office of Sciences Advanced Photon Source (APS) at Argonne National Laboratory is one of the worlds most productive X-ray light source facilities. The APS provides high-brightness X-ray beams to a diverse community of researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. These X-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nations economic, technological, and physical well-being. Each year, more than 5,000 researchers use the APS to produce over 2,000 publications detailing impactful discoveries, and solve more vital biological protein structures than users of any other X-ray light source research facility. APS scientists and engineers innovate technology that is at the heart of advancing accelerator and light-source operations. This includes the insertion devices that produce extreme-brightness X-rays prized by researchers, lenses that focus the X-rays down to a few nanometers, instrumentation that maximizes the way the X-rays interact with samples being studied, and software that gathers and manages the massive quantity of data resulting from discovery research at the APS.
This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
####
About DOE/Argonne National Laboratory
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nations first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance Americas scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energys Office of Science.
The U.S. Department of Energys Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit https://energy.gov/science.
For more information, please click here
Contacts:
Diana Anderson
DOE/Argonne National Laboratory
Copyright © DOE/Argonne National Laboratory
If you have a comment, please Contact us.
Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
News and information
![]()
New compound unleashes the immune system on metastases September 8th, 2023
![]()
Machine learning contributes to better quantum error correction September 8th, 2023
![]()
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Imaging
![]()
Quantum powers researchers to see the unseen September 8th, 2023
![]()
USTC achieved dynamic imaging of interfacial electrochemistry August 11th, 2023
![]()
The picture of health: Virginia Tech researchers enhance bioimaging and sensing with quantum photonics June 30th, 2023
Laboratories
![]()
A non-covalent bonding experience: Scientists discover new structures for unique hybrid materials by altering their chemical bonds July 21st, 2023
Govt.-Legislation/Regulation/Funding/Policy
![]()
Quantum powers researchers to see the unseen September 8th, 2023
![]()
Chloride ions from seawater eyed as possible lithium replacement in batteries of the future August 11th, 2023
![]()
Tattoo technique transfers gold nanopatterns onto live cells August 11th, 2023
![]()
The present and future of computing get a boost from new research July 21st, 2023
Possible Futures
![]()
New compound unleashes the immune system on metastases September 8th, 2023
![]()
Machine learning contributes to better quantum error correction September 8th, 2023
![]()
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Discoveries
![]()
Electronic detection of DNA nanoballs enables simple pathogen detection Peer-Reviewed Publication September 8th, 2023
![]()
Training quantum computers: physicists win prestigious IBM Award September 8th, 2023
![]()
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Announcements
![]()
Electronic detection of DNA nanoballs enables simple pathogen detection Peer-Reviewed Publication September 8th, 2023
![]()
Training quantum computers: physicists win prestigious IBM Award September 8th, 2023
![]()
Machine learning contributes to better quantum error correction September 8th, 2023
![]()
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Automotive/Transportation
![]()
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]()
Chloride ions from seawater eyed as possible lithium replacement in batteries of the future August 11th, 2023
![]()
Graphene-based Carbocatalysts: Synthesis, Properties, and ApplicationsBeyond Boundaries June 9th, 2023
![]()
Channeling mechanical energy in a preferred direction April 14th, 2023










