Home > Press > The power of going small: Copper oxide subnanoparticle catalysts prove most superior
 |
| A research concept:Subnano copper oxide particles for solvent-free aerobic oxidation of hydrocarbons |
Abstract:
Scientists at Tokyo Institute of Technology have shown that copper oxide particles on the sub-nanoscale are more powerful catalysts than those on the nanoscale. These subnanoparticles can also catalyze the oxidation reactions of aromatic hydrocarbons far more effectively than catalysts currently used in industry. This study paves the way to better and more efficient utilization of aromatic hydrocarbons, which are important materials for both research and industry.
The power of going small: Copper oxide subnanoparticle catalysts prove most superior
Tokyo, Japan | Posted on February 11th, 2020
The selective oxidation of hydrocarbons is important in many chemical reactions and industrial processes, and as such, scientists have been on the lookout for more efficient ways to carry out this oxidation. Copper oxide (CunOx) nanoparticles have been found useful as a catalyst for processing aromatic hydrocarbons, but the quest of even more effective compounds has continued.
In the recent past, scientists applied noble metal-based catalysts comprising of particles at the sub-nano level. At this level, particles measure less than a nanometer and when placed on appropriate substrates, they can offer even higher surface areas than nanoparticle catalysts to promote reactivity.
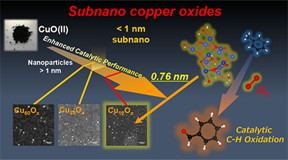
In this trend, a team of scientists including Prof. Kimihisa Yamamoto and Dr. Makoto Tanabe from Tokyo Institute of Technology (Tokyo Tech) investigated chemical reactions catalyzed by CunOx subnanoparticles (SNPs) to evaluate their performance in the oxidation of aromatic hydrocarbons. CunOx SNPs of three specific sizes (with 12, 28, and 60 copper atoms) were produced within tree-like frameworks called dendrimers (Fig. 2). Supported on a zirconia substrate, they were applied to the aerobic oxidation of an organic compound with an aromatic benzene ring.
X-ray photoelectron spectroscopy (XPS) and infrared spectroscopy (IR) were used to analyze the synthesized SNPs’ structures, and the results were supported by density functionality theory (DFT) calculations.
The XPS analysis and DFT calculations revealed increasing ionicity of the copperoxygen (CuO) bonds as SNP size decreased. This bond polarization was greater than that seen in bulk CuO bonds, and the greater polarization was the cause of the enhanced catalytic activity of the CunOx SNPs.
Tanabe and the team members observed that the CunOx SNPs sped up the oxidation of the CH3 groups attached to the aromatic ring, thereby leading to the formation of products. When the CunOx SNP catalyst was not used, no products were formed. The catalyst with the smallest CunOx SNPs, Cu12Ox, had the best catalytic performance and proved to be the longest lasting.
As Tanabe explains, “the enhancement of the ionicity of the CuO bonds with decrease in size of the CunOx SNPs enables their better catalytic activity for aromatic hydrocarbon oxidations.”
Their research supports the contention that there is great potential for using copper oxide SNPs as catalysts in industrial applications. “The catalytic performance and mechanism of these size-controlled synthesized CunOx SNPs would be better than those of noble metal catalysts, which are most commonly used in industry at present,” Yamamoto say, hinting at what CunOx SNPs can achieve in the future.
####
For more information, please click here
Contacts:
Further Information
Specially Appointed Associate Professor Makoto Tanabe
Hybrid Materials Unit
Laboratory for Chemistry and Life Science
Tokyo Institute of Technology
Tel +81-45-924-5260
Professor Kimihisa Yamamoto
Hybrid Materials Unit
Laboratory for Chemistry and Life Science
Tokyo Institute of Technology
Tel +81-45-924-5873
Contact
Public Relations Section, Tokyo Institute of Technology
Tel +81-3-5734-2975
Copyright © Tokyo Institute of Technology
If you have a comment, please Contact us.
Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
News and information
![]() New green technology from UMass Amherst generates electricity ‘out of thin air’ Renewable device could help mitigate climate change, power medical devices February 17th, 2020
New green technology from UMass Amherst generates electricity ‘out of thin air’ Renewable device could help mitigate climate change, power medical devices February 17th, 2020
Chemistry
![]() Bubble-capturing surface helps get rid of foam: Bubbly buildup can hinder many industrial processes, but a new method can reduce or even eliminate it February 12th, 2020
Bubble-capturing surface helps get rid of foam: Bubbly buildup can hinder many industrial processes, but a new method can reduce or even eliminate it February 12th, 2020
![]() A megalibrary of nanoparticles: Researchers at Penn State have developed a simple approach that could produce over 65,000 different types of complex nanoparticles January 30th, 2020
A megalibrary of nanoparticles: Researchers at Penn State have developed a simple approach that could produce over 65,000 different types of complex nanoparticles January 30th, 2020
![]() American Chemical Society names Philip Proteau as new editor-in-chief of the Journal of Natural Products January 24th, 2020
American Chemical Society names Philip Proteau as new editor-in-chief of the Journal of Natural Products January 24th, 2020
![]() American Chemical Society names Dr. James Milne head of its Publications Division January 24th, 2020
American Chemical Society names Dr. James Milne head of its Publications Division January 24th, 2020
Possible Futures
![]() New green technology from UMass Amherst generates electricity ‘out of thin air’ Renewable device could help mitigate climate change, power medical devices February 17th, 2020
New green technology from UMass Amherst generates electricity ‘out of thin air’ Renewable device could help mitigate climate change, power medical devices February 17th, 2020
Discoveries
![]() New green technology from UMass Amherst generates electricity ‘out of thin air’ Renewable device could help mitigate climate change, power medical devices February 17th, 2020
New green technology from UMass Amherst generates electricity ‘out of thin air’ Renewable device could help mitigate climate change, power medical devices February 17th, 2020
Announcements
![]() New green technology from UMass Amherst generates electricity ‘out of thin air’ Renewable device could help mitigate climate change, power medical devices February 17th, 2020
New green technology from UMass Amherst generates electricity ‘out of thin air’ Renewable device could help mitigate climate change, power medical devices February 17th, 2020
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() New green technology from UMass Amherst generates electricity ‘out of thin air’ Renewable device could help mitigate climate change, power medical devices February 17th, 2020
New green technology from UMass Amherst generates electricity ‘out of thin air’ Renewable device could help mitigate climate change, power medical devices February 17th, 2020
Energy
![]() New green technology from UMass Amherst generates electricity ‘out of thin air’ Renewable device could help mitigate climate change, power medical devices February 17th, 2020
New green technology from UMass Amherst generates electricity ‘out of thin air’ Renewable device could help mitigate climate change, power medical devices February 17th, 2020
![]() A consensus statement establishes the protocols to assess and report stability of perovskite photovoltaic devices February 1st, 2020
A consensus statement establishes the protocols to assess and report stability of perovskite photovoltaic devices February 1st, 2020
![]() Old Molecule, New Tricks: Chemistry professors develop an electrochemical method for extracting uranium, and potentially other metal ions, from solution January 24th, 2020
Old Molecule, New Tricks: Chemistry professors develop an electrochemical method for extracting uranium, and potentially other metal ions, from solution January 24th, 2020
![]() A consensus statement establishes the protocols to study stability of perovskite photovoltaic devices January 24th, 2020
A consensus statement establishes the protocols to study stability of perovskite photovoltaic devices January 24th, 2020










