| Feb 02, 2023 |
|
(Nanowerk News) Researchers at Chalmers University of Technology and partners within the Chemical Imaging Infrastructure have produced a method whereby it is possible to see at the nano level where a medicinal drug ends up in the cells and how much of it is needed for optimum treatment. The technique enables the development of new pharmaceuticals and tailored treatments for diseases that have not previously been treatable.
|
|
‘Without needing to add anything to affect the cell, we can produce unique precision at the nano level. That is not possible with comparable methods that are currently in use,’ says Per Malmberg, Director of the infrastructure and senior researcher in the Department of Chemistry and Chemical Engineering at Chalmers University of Technology.
|
|
Thanks to the comprehensive knowledge of the human genome, researchers can design more effective drugs that work by engaging specific targets in the interior of the cell. This advance also makes it necessary for drug designers to consider how their molecules behave inside the cell.
|
|
The new method, developed by the partners within the Chemical Imaging Infrastructure, is described in a recently released whitepaper. It involves enhanced cutting-edge technology and knowledge to analyse and quantify biological medicines, such as peptides and oligonucleotides, in human cells with considerable reliability.
|
 |
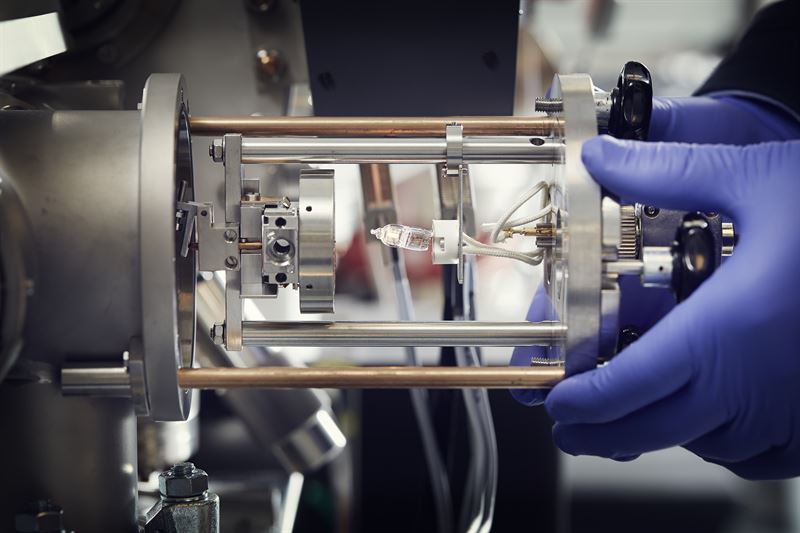
| The image shows how a sample, which sits on the round metal disk, is introduced into the nanoSIMS instrument. (Image: Anna-Lena Lundqvist, Chalmers)
|
|
The new method is based on the NanoSIMS (nanoscale secondary ion mass spectrometry) instrument developed by CAMECA, which can measure and image molecules at high resolution on the nanoscale and has been available at the chemical imaging infrastructure since 2015. The instrument has been widely adopted by the scientific community for research, but it has not yet been applied to the development of medicinal drugs.
|
|
‘Compared with similar techniques, the NanoSIMS methodology provides much faster and more accurate answers. With our technique, a drug project can receive an answer within about four weeks, and there are good opportunities to reduce the time even further, says Per Malmberg.
|
Significance for unmet medical needs
|
|
So far, researchers have worked with cultured cells, but the technique can also be used to examine tissue. In the long term, it could also be used to investigate what happens in individual cells in an organ where the drug is expected to act. This could provide a key to a deeper understanding of, for example, neurodegenerative diseases, such as ALS or Parkinson’s disease, and cancer.
|
|
The pharmaceutical industry has a significant need to develop and apply methodologies for nanoscale quantification of drug molecules and their distribution at the sub-cellular level.
|
|
‘I am extremely pleased that we have succeeded in imaging medicinal drugs in cells. There are many things that can happen to a drug once it enters the cell. Now that we can make observations at this level for the first time, we can obtain critical information that will help us design drugs for diseases that have not previously been treatable,’ says Michael Kurczy, Associate Principal Scientist at AstraZeneca.
|
Collaboration key to new results
|
|
Researchers at Chalmers University of Technology and the University of Gothenburg are responsible for the development, in collaboration with AstraZeneca, AstraZeneca’s BioVentureHub and the company CAMECA. When the infrastructure partners’ collective knowledge and expertise in terms of the preparation and measurement of samples were combined, results were achieved that would not have been possible without such collaboration.
|
|
‘It is a great opportunity for researchers, especially young ones, to work at the interface of academia, industry and engineering. The synergy between the developers’ direct insight into the industry’s needs and problems, and the researchers’ expertise and ideas on how they could be resolved, has been crucial in enabling us to present new, valuable tools, which will lead to a significant improvement in drug development processes and therefore the quality of people’s lives,’ says Thi Ngoc Nhu Phan, Assistant Professor at the University of Gothenburg.
|
About the Chemical Imaging infrastructure
|
|
Chemical imaging is a comprehensive research infrastructure, a primary focus of which is high-resolution mass spectrometry imaging (MSI). The infrastructure offers a wide range of tools for analytical questions in materials science, earth sciences and life sciences. The infrastructure belongs to the Department of Chemistry and Chemical Engineering at Chalmers and the Department of Chemistry and Molecular Biology at the University of Gothenburg.
|
About NanoSIMS
|
|
There are different types of mass spectrometry where the technique is refined and continuously improved, broadening the range of applications. NanoSIMS, which is a development of what is known as MSI-SIMS technology, enables molecular imaging at a very high resolution inside cells and tissues.
|
Scientific articles and white paper related to the research
|
|
Becquart et al., 2022: Intracellular Absolute Quantification of Oligonucleotide Therapeutics by NanoSIMS | Analytical Chemistry
|
|
Thomen et al. 2020: Subcellular Mass Spectrometry Imaging and Absolute Quantitative Analysis across Organelles | ACS Nano
|