| Feb 28, 2023 |
|
(Nanowerk News) One of the most expensive steps in manufacturing protein drugs such as antibodies or insulin is the purification step: isolating the protein from the bioreactor used to produce it. This step can account for up to half of the total cost of manufacturing a protein.
|
|
In an effort to help reduce those costs, MIT engineers have devised a new way to perform this kind of purification. Their approach, which uses specialized nanoparticles to rapidly crystallize proteins in a microfluidic device, could help to make protein drugs more affordable and accessible, especially in developing countries.
|
 |
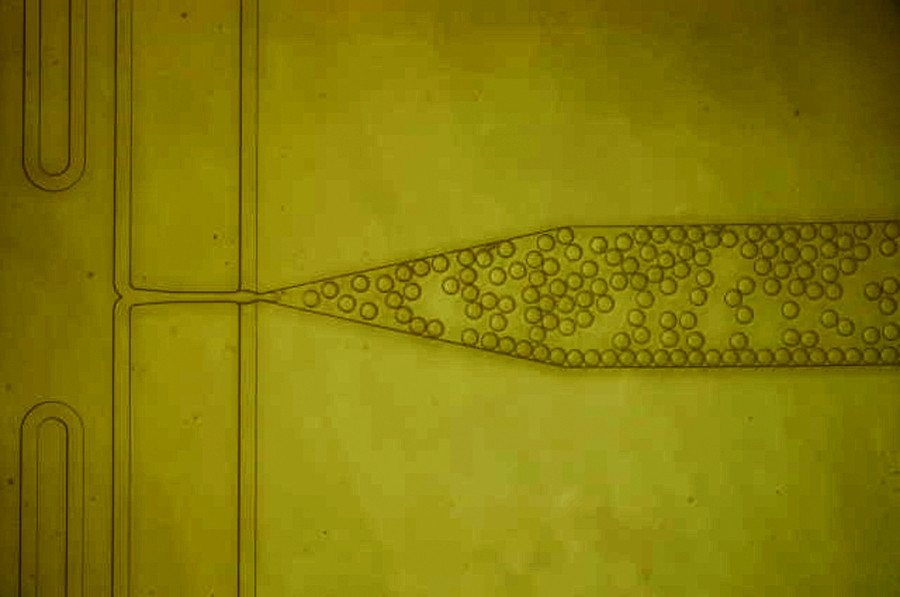
| A microfluidic device was designed to combine protein solution with nanoparticles and then form thousands of tiny, identical droplets. Inside each of these droplets, the proteins interact with the nanoparticles, which help them to form protein crystals. (Image courtesy of the researchers)
|
|
“This work uses bioconjugate-functionalized nanoparticles to act as templates for enhancing protein crystal formation at low concentrations,” says Kripa Varanasi, a professor of mechanical engineering at MIT and the senior author of the new study. “The goal is to reduce the cost so that this kind of drug manufacturing becomes affordable in the developing world.”
|
|
The researchers demonstrated that their approach can be used to crystallize lysozyme (an antimicrobial enzyme) and insulin. They believe it could also be applied to many other useful proteins, including antibody drugs and vaccines.
|
|
MIT graduate student Caroline McCue is the lead author of the study, which appears in the journal ACS Applied Materials and Interfaces (“Enhancing Protein Crystal Nucleation Using In Situ Templating on Bioconjugate-Functionalized Nanoparticles and Machine Learning”). Henri-Louis Girard PhD ’20 is also an author of the paper.
|
Protein purification
|
|
Antibodies and other protein drugs are part of a growing class of drugs known as biologics, which also include molecules such as DNA and RNA, as well as cell-based therapies. Most protein drugs are produced by living cells such as yeast in large bioreactors.
|
|
Once these proteins are generated, they have to be isolated from the reactor, which is usually done through a process called chromatography. Chromatography, which separates proteins based on their size, requires specialized materials that make the process very expensive.
|
|
Varanasi and his colleagues decided to try a different approach, based on protein crystallization. Researchers often crystallize proteins to study their structures, but the process is considered too slow for industrial use and doesn’t work well at low concentrations of protein. To overcome those obstacles, Varanasi’s lab set out to use nanoscale structures to speed up the crystallization.
|
|
In previous work, the lab has used nanoscale features to create materials that repel water or to modify interfaces for injecting highly viscous biologic drugs. In this case, the researchers wanted to adapt nanoparticles so that they could locally increase the concentration of protein at the surface and also provide a template that would allow the proteins to align correctly and form crystals.
|
|
To create the surface they needed, the researchers coated gold nanoparticles with molecules called bioconjugates — materials that can help form links between other molecules. For this study, the researchers used bioconjugates called maleimide and NHS, which are commonly used for tagging proteins for study or attaching protein drugs to drug-delivering nanoparticles.
|
 |
| In this time-lapse video, protein crystals form more rapidly on nanoparticles functionalized with molecules called bioconjugates (top). The nanoparticles at the bottom do not have the bioconjugate molecules. (Image courtesy of the researchers)
|
|
When solutions of proteins are exposed to these coated nanoparticles, the proteins accumulate at the surface and bind to the bioconjugates. Furthermore, the bioconjugates compel the proteins to align themselves with a specific orientation, creating a scaffold for additional proteins to come along and join the crystal.
|
|
The researchers demonstrated their approach with lysozyme, an enzyme whose crystallization properties have been well studied, and insulin. They say it could also be applied to many other proteins.
|
|
“This is a general approach that could be scaled to other systems as well. If you know the protein structure that you’re trying to crystallize, you can then add the right bioconjugates that will force this process to happen,” Varanasi says.
|
Rapid crystallization
|
|
In their studies with lysozyme and insulin, the researchers found that crystallization occurred much faster when the proteins were exposed to the bioconjugate-coated nanoparticles, compared to bare nanoparticles or no nanoparticles. With the coated particles, the researchers saw a sevenfold reduction in the induction time — how long it takes for crystals to begin forming — and a threefold increase in the nucleation rate, which is how quickly the crystals grow once started.
|
|
“Even at low protein concentrations, we see a lot more crystals forming with these bioconjugate-functionalized nanoparticles,” McCue says. “The functionalized nanoparticles reduce the induction time so much because these bioconjugates are providing a specific site for the proteins to bind. And because the proteins are aligned, they can form a crystal faster.”
|
|
In addition, the team used machine learning to analyze thousands of images of crystals. “Protein crystallization is a stochastic process, so we needed to have a huge dataset to be able to really measure whether our approach was improving the induction time and nucleation rate of crystallization. With so many images to process, machine learning is the best way to be able to determine when crystals are forming in each image without having to go through and manually count each one,” McCue says.
|
|
This project is part of a Bill and Melinda Gates Foundation effort to make biologic drugs, such as prophylactic antibodies that have been shown to prevent malaria in clinical trials, more widely available in developing nations.
|
|
The MIT team is now working on scaling up the process so that it could be used in an industrial bioreactor, and demonstrating that it can work with monoclonal antibodies, vaccines, and other useful proteins.
|
|
“If we can make it easier to manufacture these proteins anywhere, then everyone in the world can benefit,” Varanasi says. “We are not saying that this is going to be solved tomorrow because of us, but this is a small step that can contribute to that mission.”
|