| Sep 26, 2022 |
|
(Nanowerk News) In the last decades, a new synthetic approach has been developed, generally termed as “on-surface synthesis” that substantially departs from standard wet-chemistry. Instead of the three-dimensional space of solvents in the latter, the environment of the reactants in this new approach are well-defined two-dimensional solid surfaces that are typically held under vacuum conditions. These differences have allowed the successful synthesis of a great variety of molecular structures that could not be obtained by conventional means.
|
|
Among the structures that are raising particular interest, we find carbon-nanostructures with zigzag-shaped edges, which endow the materials with exciting electronic and even magnetic properties of potential interest for a great variety of applications that include quantum technologies.
|
|
An important downside of these materials, however, is that they often lack sufficient chemical stability to withstand air exposure. That is why environments like vacuum are used to make the synthesis possible.
|
|
Unfortunately, for their ultimate implementation in actual devices, these structures need to be manipulated and transferred out of the vacuum, which would degrade the materials and therefore jeopardize their potential utilization.
|
|
This brings up the need to conceive new strategies for the device fabrication processes. In conventional chemistry, protection/deprotection strategies are commonly applied to overcome stability problems. However, it remained to be tested whether such protection chemistry strategies could also be applied in “on-surface synthesis”.
|
|
In this work, an international team from DIPC and CFM (CSIC-UPV/EHU) in San Sebastian, CIQUS – Universidade de Santiago de Compostela, Czech Academy of Sciences (Prague), Palacký University (Olomouc), Ikerbasque (Basque Country) and CINN (CSIC-UNIOVI-PA) in El Entrego, performed such tests with narrow stripes of graphene nanoribbons featuring a large density of zigzag-shaped edges.
|
 |
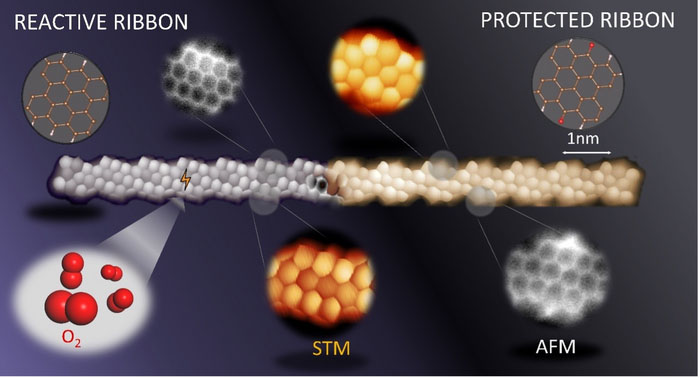
| Scanning probe microscopy image of a reactive (left) and protected (right) graphene nanoribbon. (Image: DIPC | CFM | FZU | CiQUS | CATRIN)
|
|
The work, now published in Nature Chemistry (“Circumventing the stability problems of graphene nanoribbon zigzag edges”), presents two related but complementary methods to apply the protection/deprotection strategy to the reactive zigzag edge segments of nanographenes.
|
|
In particular, they have demonstrated the usage of atomic hydrogen as a means of protecting the nanostructured graphene from the oxidising effects of the atmosphere. Afterwards, the nanostructures were easily dehydrogenated and converted back to their original form via annealing. An alternative approach further allowed them to convert an air-stable, chemically modified form of the graphene nanostructures with protective ketone side groups, into the molecules of interest.
|
|
The implications of these results are far reaching. The demonstrated protection/deprotection strategy is expected to be similarly applicable to graphene nanostructures with zigzag edge segments different from those probed here. It thus opens new doors for the conception of approaches to integrate carbon nanostructures into devices and may thereby bring the exploitation of the unique characteristics of their zigzag edges a step closer to scalable applications, a grand scientific challenge that cuts across physics, chemistry, materials science and engineering.
|