(Nanowerk News) Scientists crafting a nickel-based catalyst used in making hydrogen fuel built it one atomic layer at a time to gain full control over its chemical properties. But the finished material didnt behave as they expected: As one version of the catalyst went about its work, the top-most layer of atoms rearranged to form a new pattern, as if the square tiles that cover a floor had suddenly changed to hexagons.
But thats ok, they reported today, because understanding and controlling this surprising transformation gives them a new way to turn catalytic activity on and off and make good catalysts even better.
The research team, led by scientists from Stanford University and the Department of Energys SLAC National Accelerator Laboratory, described their study in Nature Materials (“Tuning electrochemially driven surface transformation in atomically flat LaNiO3 thin films for enhanced water electrolysis”).
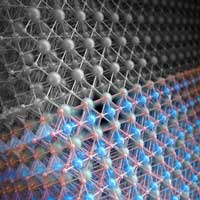
 An illustration combines two possible types of surface layers for a catalyst that performs the water-splitting reaction, the first step in making hydrogen fuel. The gray surface, top, is lanthanum oxide. The colorful surface is nickel oxide; a rearrangement of its atoms while carrying out the reaction made it twice as efficient, a phenomenon researchers hope to harness to design better catalysts. Lanthanum atoms are depicted in green, nickel in blue and oxygen in red. (Image: SLAC)
Catalysts can change very quickly during the course of a reaction, and understanding how they transform from an inactive phase to an active one is crucial to designing more efficient catalysts, said Will Chueh, an investigator with the Stanford Institute for Materials and Energy Sciences (SIMES) at SLAC who led the study. This transformation gives us the equivalent of a knob we can turn to fine-tune their behavior.
An illustration combines two possible types of surface layers for a catalyst that performs the water-splitting reaction, the first step in making hydrogen fuel. The gray surface, top, is lanthanum oxide. The colorful surface is nickel oxide; a rearrangement of its atoms while carrying out the reaction made it twice as efficient, a phenomenon researchers hope to harness to design better catalysts. Lanthanum atoms are depicted in green, nickel in blue and oxygen in red. (Image: SLAC)
Catalysts can change very quickly during the course of a reaction, and understanding how they transform from an inactive phase to an active one is crucial to designing more efficient catalysts, said Will Chueh, an investigator with the Stanford Institute for Materials and Energy Sciences (SIMES) at SLAC who led the study. This transformation gives us the equivalent of a knob we can turn to fine-tune their behavior.
Splitting water to make hydrogen fuel
Catalysts help molecules react without being consumed in the reaction, so they can be used over and over. Theyre the backbone of many green-energy devices. This particular catalyst, lanthanum nickel oxide or LNO, is used to split water into hydrogen and oxygen in a reaction powered by electricity. Its the first step in generating hydrogen fuel, which has enormous potential for storing renewable energy from sunlight and other sources in a liquid form thats energy-rich and easy to transport. In fact, several manufacturers have already produced electric cars powered by hydrogen fuel cells. But this first step is also the most difficult one, said Michal Bajdich, a theorist at the SUNCAT Center for Interface Science and Catalysis at SLAC, and researchers have been searching for inexpensive materials that will carry it out more efficiently. Since reactions take place on a catalysts surface, researchers have been trying to precisely engineer those surfaces so they promote only one specific chemical reaction with high efficiency. A new study shows how tweaking the surface layer of a catalyst can make it work better. This particular catalyst is used to split water, the first step in making hydrogen fuel. It consists of alternating layers of materials rich in nickel (blue spheres) and lanthanum (green spheres; the red spheres represent oxygen atoms). When the material is grown at relatively cool temperatures so a nickel-rich layer is on top (left), the atoms on that surface layer rearrange during the water-splitting reaction (middle) in a way that allows them to carry out the reaction more efficiently (right). This surprising result gives scientists a new way to tune catalytic activity and engineer better catalysts. (Image: Tomas Duchon/Forschungszentrum Juelich)
A new study shows how tweaking the surface layer of a catalyst can make it work better. This particular catalyst is used to split water, the first step in making hydrogen fuel. It consists of alternating layers of materials rich in nickel (blue spheres) and lanthanum (green spheres; the red spheres represent oxygen atoms). When the material is grown at relatively cool temperatures so a nickel-rich layer is on top (left), the atoms on that surface layer rearrange during the water-splitting reaction (middle) in a way that allows them to carry out the reaction more efficiently (right). This surprising result gives scientists a new way to tune catalytic activity and engineer better catalysts. (Image: Tomas Duchon/Forschungszentrum Juelich)