| Jan 06, 2023 |
|
(Nanowerk News) In a warming world, coal can often seem the “bad guy.” But we can do other things with coal besides burn it. A team at Ohio University used PSC’s Bridges-2 system to carry out a series of simulations showing how coal might eventually be converted to valuable — and carbon-neutral — materials like graphite and carbon nanotubes.
|
 |
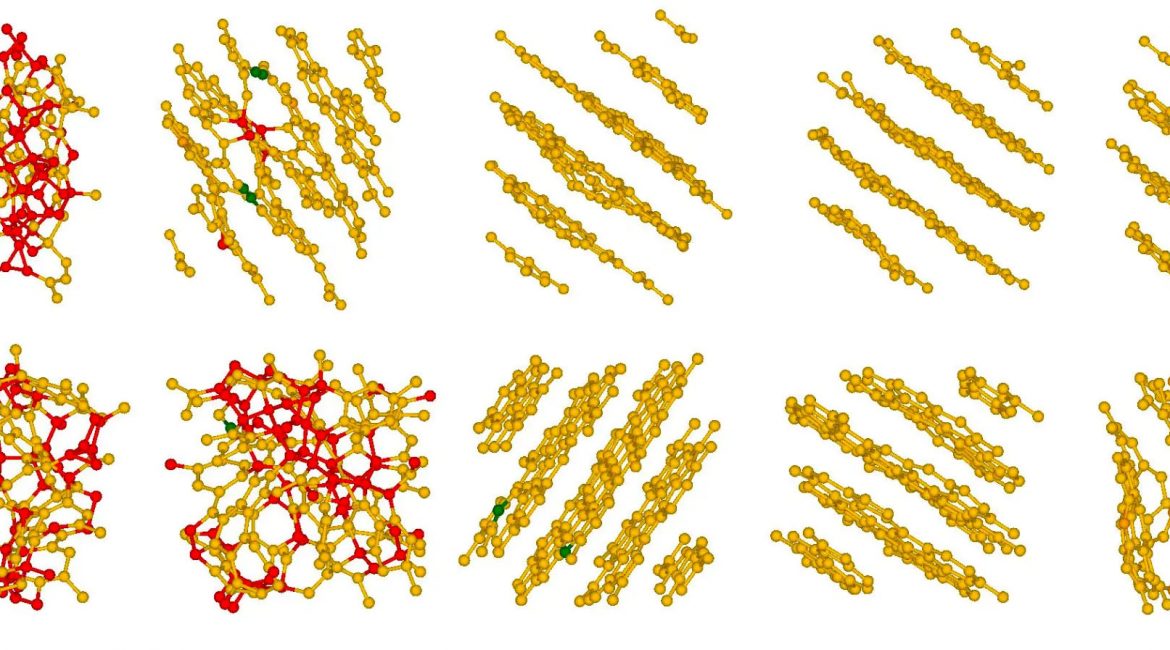
| Time series (left to right) showing two examples of how the random carbons in the artificial “coal” coalesce into graphite-like sheets under pressure and heat. The sheets aren’t perfectly flat because of the formation of a small number of five- and seven-member rings among the six-member rings. (Image: Pittsburgh Supercomputing Center)
|
Why it is important
|
|
Coal gets some bad press these days. Climate scientists predict a rise in average global temperatures of between 2 and 10 degrees Fahrenheit by the year 2100. The possibility of drastic changes to weather patterns, crop growth, and sea levels calls our heavy use of carbon-based fuels like coal into question.
|
|
But it doesn’t have to be that way.
|
|
Powering our vehicles with electricity can reduce carbon emissions directly. The shift could also allow us to charge them using carbon-neutral energy sources. The kicker is that each Tesla model S’s lithium-ion batteries require some 100 pounds of graphite. And scientists have known for generations that, at least in theory, you can convert coal to graphite if you put it under enough pressure at a high enough temperature.
|
|
To explore how coal can be converted into valuable materials like graphite, David Drabold and his team at Ohio University decided to simulate the substances in computer software. To recreate the chemical conversion virtually, they turned to the Bridges-2 advanced research computer at PSC. Bridges-2 is the Pittsburgh Supercomputing Center’s flagship supercomputer, funded by the National Science Foundation.
|
How PSC helped
|
|
Pure graphite is a series of sheets made up of six-carbon rings. A special type of chemical bond called aromatic bonds holds these carbons together.
|
|
In aromatic bonds, pi electrons float above and below the rings. These “slippery” electron clouds cause the sheets to slide easily past each other. Pencil “lead” — a low-grade form of graphite — leaves a mark on paper because the sheets slip off of each other and stick to the paper.
|
|
Aromatic bonds have another virtue, important in electronic technology. The pi electrons move easily from ring to ring and sheet to sheet. This makes graphite conduct electricity, even though it’s not a metal. It’s the ideal material for an anode, the positive pole of a battery.
|
|
Coal, by comparison, is messy chemically. Unlike the strictly two-dimensional nature of a graphite sheet, it has connections in three dimensions. It also contains hydrogen, oxygen, nitrogen, sulfur, and other atoms that might disrupt graphite formation.
|
|
To begin their studies, Drabold’s team created a simplified “coal” that consisted of only carbon atoms in random positions. By exposing this simplified coal to pressure and high temperature — about 3,000 Kelvin, or nearly 5,000 Fahrenheit — they could take a first step in studying its conversion to graphite.
|
|
At first, the Ohio scientists carried out their simulations using basic physical and chemical principles via density functional theory. This accurate but calculation-heavy approach required many parallel computations — a strength of Bridges-2’s more than 30,000 computing cores. Later, they shifted their calculations to a new software tool, GAP (Gaussian approximation potential) designed by collaborators at the University of Cambridge and the University of Oxford in England. GAP uses a type of artificial intelligence called machine learning to carry out essentially the same computations much more quickly. Graduate students Rajendra Thapa and Ugwumadu traded off on leading the initial computational work.
|
|
Their results were more complicated and simpler than the team had expected. The sheets did form. But the carbon atoms didn’t entirely develop simple, six-carbon rings. A fraction of the rings had five carbons; others had seven.
|
|
The non-six-carbon rings posed an interesting wrinkle, in more ways than one. While six-carbon rings are flat, five- and seven-membered carbon rings pucker, but in opposite senses of “positive and negative curvature.” The scientists might have expected these puckers to ruin the formation of the graphite sheets. But sheets formed anyway, possibly because pentagons and heptagons balanced each other in the simulations. The sheets were technically amorphous graphite because they weren’t purely six-ringed. But again, they formed layers.
|
|
In another series of simulations, Ugwumadu followed up on his work with Thapa to study molecules rather than solids. The conditions in these sims caused the sheets to curve in on themselves. Instead of sheets, they formed nested amorphous carbon nanotubes (CNTs) — a series of single-atomic-layer tubes, one inside another. CNTs have been hot in materials science lately, as they are in effect tiny wires that can be used to conduct electricity at incredibly small scales. Other promising applications of CNTs include fuel cell catalysis, production of supercapacitors and lithium-ion batteries, electromagnetic interference shielding, biomedical sciences, and nano-neuroscience.
|
|
One important facet of the CNT work was that Ugwumadu studied how amorphous wrinkles in the tube walls affect movement of electricity through the structure. In materials science, every “bug” is also a “feature:” engineers may be able to use such irregularities to tune the behavior of a given CNT to match the exact requirements needed in a new electronic device.
|
The scientists published their results in two papers, one on the formation of the amorphous graphite sheets in the journal Physical Review Letters (“Ab Initio Simulation of Amorphous Graphite
“), and one about the CNTs in Physica Status Solidi B (“Formation of amorphous carbon multi-walled nanotubes from random initial configurations”). Another, on how the five- and seven-member rings fit into the sheets, is in press in the European Journal of Glass Science and Technology.
|
|
The Ohio team continues to study the conversion of carbon atoms to graphite and related materials. Another ongoing project is simulating amorphous nested fullerenes, soccer-ball-shaped structures that are of scientific interest, especially in nano-neuroscience. They also published a paper on the fullerenes in November 2022. The team is also investigating using Bridges-2’s powerful graphics processing units, which potentially could speed their ML-based VAST computations, to make more complicated materials like real-world coal accessible to their simulations.
|